Latest Research
- 2017.12.18
- Osakada Group
Synthesis of Optically Active Polystyrene by Asymmetric Polymerization of Styrene Catalyzed by Monophosphine Pd Complexes
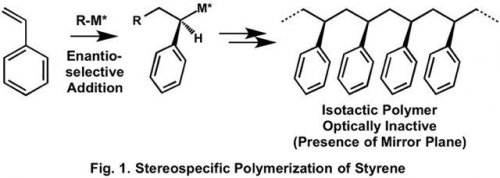
Optically active polymers are abundant in the natural world, and their chemical synthesis through polymerization of prochiral monomers has been extensively studied. Stereospecific addition polymerization of prochiral styrene generally produces optically inactive polystyrene because of the presence of mirror plane in the produced stereoregular polystyrene (Fig. 1).1)
There have been a few methods for synthesis of optically active polystyrene, such as utilization of bis(styrene) with optically active tether2) or stereospecific oligomerization by optically active catalyst in the presence of chain transfer agent.3) However, direct synthesis of optically active polystyrene by homopolymerization of styrene has been still challenging.
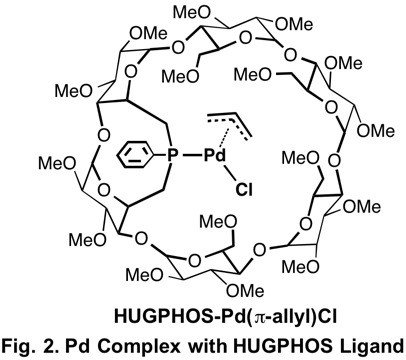
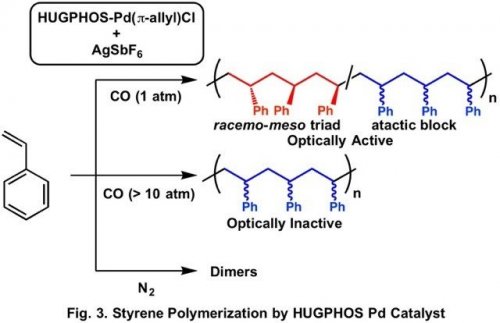
We found that Pd complex with HUGPHOS ligand, which has optically active cyclodextrin structure, promotes polymerization of styrene under CO atmosphere.4) Stereoregularity of the produced polystyrene is not so high but the polystyrene shows optical activity. The pressure of CO is critical in the formation of the optically active polystyrene. Although optically active polystyrene is formed under 1 atm of CO, the polystyrene obtained under higher CO pressure is not optically active. The polymerization under N2 atmosphere affords dimers of styrene due to frequent chain transfer reaction.
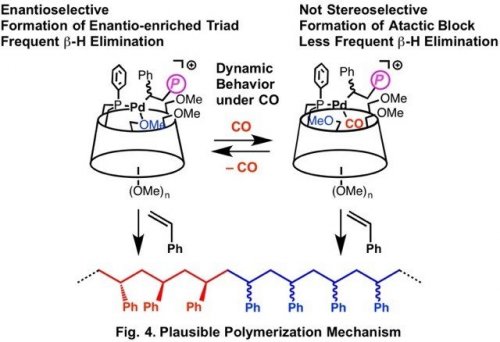
Detailed analysis of the repeating structure of the optically active polystyrene obtained under 1 atm of CO indicates the presence of increased amount of racemo-meso triad structure compared to that of the optically inactive polymer obtained under increased CO pressure. The analysis of the HUGPHOS Pd complex under CO atmosphere showed that CO is weakly coordinated to the Pd center and reversibly undergoes dissociation and coordination to the Pd center during the polymerization. The Pd species with coordinated CO does not cause chain transfer easily, but its stereoselectivity in chain growth is low. On the contrary, the Pd species without coordination of CO can allow enantioselective chain growth, whereas it easily undergoes chain transfer via b-hydrogen elimination. Under appropriate pressure of CO, reversible coordination and dissociation of CO leads to the equilibrated mixture of those two species, which enables formation of the polystyrene containing alternating enantio-enriched racemo-meso triad and atactic block with sufficient molecular weight. Such dynamic behavior of the optically active complex would enable synthesis of optically active polymers from various prochiral monomers.
This is a collaborated work with Dr. Matthieu Jouffroy, Prof. Dominique Armspach, and Prof. Dominique Matt in Strasbourg University.
1) Y. Okamoto, T. Nakano, Chem. Rev. 1994, 94, 349-372.
2) G. Wulff, P. K. Dhal, Angew. Chem. Int. Ed. 1989, 28, 196-198.
3) K. Beckerle, R. Manivannan, B. Lian, G.-J. M. Meppelder, G. Raabe, T. P. Spaniol, H. Ebeling, F. Pelascini, R. Mülhaupt, J. Okuda, Angew. Chem. Int. Ed. 2007, 46, 4790-4793.
4) M. Jouffroy, D. Armspach, D. Matt, K. Osakada, D. Takeuchi, Angew. Chem. Int. Ed. 2016, 55, 8367-8370.