Latest Research
- 2016.01.19
Development of High-Conductive Organomtallic Molecular Wires
In recent years, there has been increasing interest in molecular electronics because of the difficulty in and the large cost for downsizing silicon-based semiconductors. Single molecule devices are essential components for molecular electronics. In particular, organometallic complexes with two redox active metal units linked by a p-conjugated spacer are considered to be nano-scale conducting wires. In order to construct an efficient organometallic wire, M-BL-M, a variety of transition metal fragments (M) and bridging ligands (BL) have been examined. We have been studying organometallic molecular wires with various bridging lingad [1] and found that molecular wires with heteroaromatic rings, such as pyrrolyl and furanyl rings, show good wire-like performance.[2] For development of better molecular-wire systems, we anticipated that incorporation of a much more extended aromatic system into the bridging ligand should change the electronic structure of the organometallic wire to lead to an enhancement of the interaction between the metal fragments and the bridging ligand. Herein, we report synthesis of a molecular wire with the electron-rich -C≡C-RuCp*(dppe) fragments linked by a free-base porphyrin (Fig. 1) and its wire-like performance.[3]
Cyclic voltammograms of the porphyrin wire show two successive reversible waves which are attributed to two Ru centers. According to the separation between the first two redox waves (DE), the comproportionation constant (KC) is determined to be 1.8 x 105. For the monocationic species, intervalence charge transfer (IVCT) bands are observed in the NIR region. According to the IVCT bands, the electronic coupling Vab are determined to be 2644 cm-1. KC and Vab values are much larger than those found for molecular wire diethynylbenzene complexes (KC = 8.0 x 104, Vab = 617 cm-1, dRu-Ru ~ 1.2 nm).[4] Therefore, the porphyrin wire shows wire-like performance better than that of the diethnylbenzene wire, despite the metal-metal distance is much longer than that for the porphyrin wire (dRu-Ru ~ 1.6 nm).
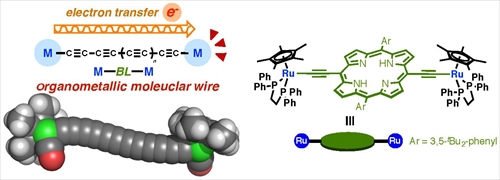
Figure1. The organometallic molecular wire and the dinuclear Ru porphyrin wire.
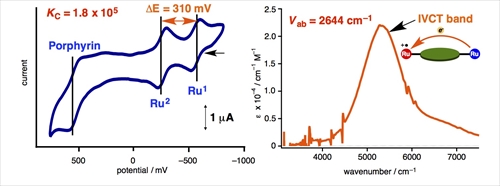
Figure 2. Cyclic voltammograms of the porphyrin Ru wire (left) and NIR spectrum of the monocationic Ru porphyrin wire (right).
To clarify the reasons why the porphyrin wire shows the wire-like performance better than the benzene wire, DFT calculations have been conducted for the model complexes (Fig. 3). For the monocationic species, the sum of the spin densities over the diethynylporphyrin part (0.73e) is larger than that of the diethynylbenzene part (0.59e), indicating more extensive delocalization of the radical cation of the porphyrin wire. Ru-C bond distance of the neutral porphyrin wire is shorter than that of the neutral benzene wire, suggesting that the p-back donation from the metal centers to the bridging ligand becomes significant as the size of the p-system in the bridging ligand increases. Therefore, high-lying HOMO and low-lying LUMO of the porphyrin ligands is the key of the good wire-like performance of the porphyrin wire.
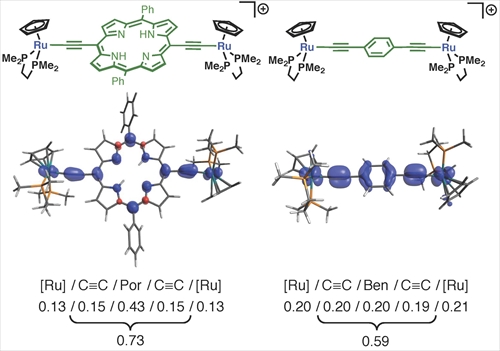
Figure 3. Plot and numerical summary of the spin density distribution in model complexes.
In summary, we have developed the high-conductive organoemtallic molecular wire with a diethynylporphyrin linker. Development of functional molecular wires and conductance measurements between metal electrodes are in progress.
References
1. M. Akita, T. Koike, Dalton Trans., 2008, 3523-3530
2. Y. Tanaka, T. Ishisaka, T. Koike, M. Akita, Polyhedron, 2015, 86, 105-110. (Prof. Claude Lapinte special issue)
3. Y. Tanaka, M. Ono, M. Akita, J. Porphyrins Phthalocyanines, 2015, 19, 442-450. (Prof. Shunichi Fukuzumi special issue)
4. M. A. Fox, B. Le Guennic, R. L. Roberts, D. A. Brue, D. S. Yufit, J. A. K. Howard, G. Manca, J.-F. Halet, F. Hartl, P. J. Low, J. Am. Chem. Soc., 2011, 133, 18433-18446.



