Latest Research
- 2014.02.27
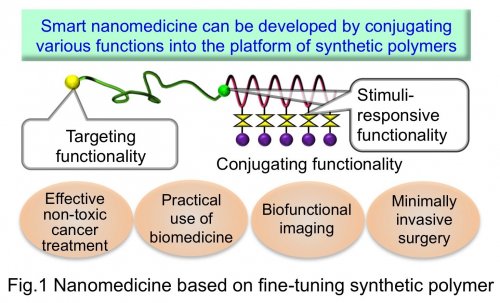
Development of nanomedicine based on fine-tuning synthetic polymers
Development of new drugs depends on the drug discovery & development processes including the target discovery, combinatorial synthesis and screening of drug candidates and identification of lead compounds. Furthermore, new drugs should pass the efficacy / safety test in animals and clinical evaluation in the patients for their approval for clinical use. Only 0.011% of drug candidates can progress to clinical evaluation and finally 0.003% can be approved. Also, the current drug discovery & development processes require huge costs reaching several billion US dollars and time more than ten years. Furthermore, it is getting more difficult to obtain new drugs by such processes.
Recent advances in biotechnology allow to develop various functional molecules including targeting molecules such as aptamers, peptides and antibodies, and their application in medicine is strongly demanded. On the other hand, the development of stimuli-responsive smart materials has recently been receiving increasing attention in the fields of materials science / nanotechnology. These smart materials are also expected to be used in medicine. However, there seems to be an obstacle for such applications.
To overcome the above-mentioned situations, we have studied the new concept of drug development based on fine-tuning synthetic polymers. In the design of polymeric drugs, the active moieties (drugs) and various functional molecules are conjugated to the platform of synthetic polymers, leading to enhancing drug functions. Importantly, the basis of developing such polymeric drugs is the precision polymerization (living polymerization) technology to control the primary structure (molecular weight, compositions and position of functional groups) of synthetic polymers. In the Polymer Chemistry Division (Prof. Nishiyama's Laboratory) in Chemical Resources Laboratory, we integrate the targeting functionality, stimuli-responsive functionality and drug conjugating functionality into a single polymer chain, thereby aiming to realize multifunctional polymeric drugs (smart nanomedicine). Our targets include realization of effective but non-toxic cancer treatment, practical use of emerging biomedicine, biofunctional imaging and minimally invasive surgery in combination with medical instruments (Fig.1).
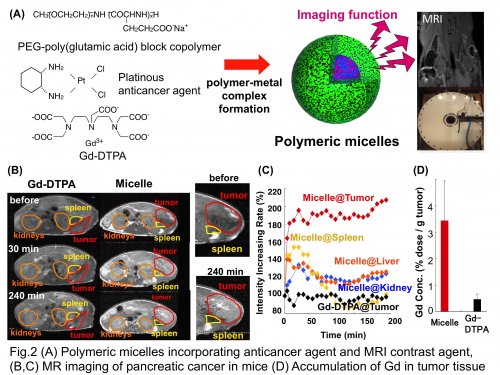
In this article, we would like to introduce nanomedicine with imaging functionality (visible nanomedicine). In current chemotherapy, there is no method to estimate the drug distribution in the body and adequately evaluate the patients' response to the drug treatment. Consequently, the current chemotherapy sometimes results in ineffective therapeutic outcome and ultimately past cure. The precise monitoring of the drug distribution in each cancer patient would allow clinicians to anticipate the therapeutic process, and furthermore, early feedback of the drug response will be helpful for customizing his/her treatment protocols for safe and successful chemotherapy. However, as an approach for drug visualization, direct conjugation of imaging contrast agents to drug molecules compromises the biodistribution and biological activity of the therapeutic entities. In contrast, integration of imaging functionality into polymeric drugs does not affect the biodistribution and biological activity, thus offering a promising platform for cancer diagnosis and therapy. To realize visible nanomedicine, we I developed a new class of polymeric micelles that simultaneously incorporate platinous anticancer agent and gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA, a clinically approved magnetic resonance imaging (MRI) contrast agent) through the metal complexation with engineered block copolymers (Fig.2 A). Incorporation of Gd-DTPA into the micellar structure resulted in 24-times enhancement of the longitudinal relaxivity r1 (MRI contrast enhancement ability) due to the slow molecular reorientation effect. Also, polymeric micelles released Gd-DTPA under a physiological condition, followed by rapid renal clearance, avoiding the problems of long-term accumulation of toxic Gd3+ ion in the body. In animal experiments, polymeric micelles successfully visualized the orthotopically inoculated pancreatic cancer by MRI (Fig.2 B and C). Worth noting is that malignant tumors are characterized by leaky vasculature and impaired lymphatic drainage, allowing polymeric drugs to effectively accumulate in the tumor tissue. Indeed, polymeric micelles showed 7-times higher Gd accumulation in the tumor compared with free Gd-DTPA (Fig.2 D). It is expected that visible nanomedicine will improve the safety and effectiveness of cancer treatment, offering novel personalized medicine based on diagnostic imaging.
1) P. Mi, H. Cabral, D. Kokuryo, M. Rafi, Y. Terada, I. Aoki, T. Saga, T. Ishii, N. Nishiyama, K. Kataoka, Gd-DTPA-loaded polymer-metal complex micelles with high relaxivity for MR cancer imaging. Biomaterials 34 (2) 492-500 (2013)
2) H. Cabral, N. Nishiyama, K. Kataoka, Supramolecular nanodevices: From design validation to theranostic nanomedicine. Acc. Chem. Res. 44 (10) 999-1008 (2011)
3) S. Kaida, H. Cabral, M. Kumagai, A. Kishimura, Y. Terada, M. Sekino, I. Aoki, N. Nishiyama, T. Tani, K. Kataoka, Visible drug delivery by supramolecular nanocarriers directing to single-platformed diagnosis and therapy of pancreatic tumor model. Cancer Res. 70 (18) 7031-7041 (2010)