Latest Research
- 2014.09.22
Development of Photocatalytic Oxidative Trifluoromethylation of Alkenes
-Simple and Short Synthesis of Useful Organofluorine Compound-
Trifluoromethylated ketones are useful building blocks for organic compounds with a trifluoromethyl group. A new and simple synthesis of ketones with a trifluoromethyl substituent in the α-position proceeds through a one-pot photoredox-catalyzed trifluoromethylation-oxidation sequence of alkenes. Dimethyl sulfoxide (DMSO) serves as a key and mild oxidant under these photocatalytic conditions. Furthermore, an iridium photocatalyst, fac-[Ir(ppy)3 ] (ppy=2-phenylpyridine), turned out to be crucial for the present photoredox process.1
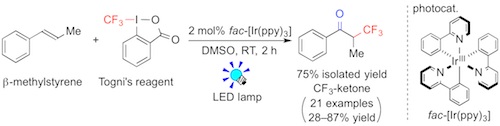
Fig.1 Photocatalytic oxidative trifluoromethylation of alkenes
The reaction of β-methylstyrene with Togni's reagent in the presence of the Ir photocatalyst (2 mol%) in DMSO solvent under visible light irradiation (LED lamp) afforded the CF3-ketone in 75% isolated yield (2 h) (Fig. 1). The present oxidative trifluoromethylation of alkenes exhibited broad scope (21 examples). In general, electrophilic or radical trifluoromethylation of enolates, which were prepared from the corresponding carbonyl compounds in advance, provides access to valuable α-trifluoromethylated carbonyl compounds. Examples of the "direct" oxidative trifluoromethylation of alkenes, which are abundant and commonly used feedstocks, have been limited so far, but should be promising way (Fig. 2).
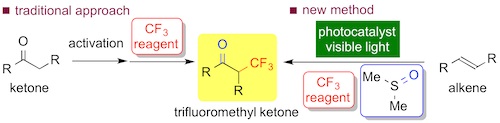
Fig. 2 A comparison between the traditional approach and new method for oxidative trifluoromethylation of alkenes using photocatalyst
A feature of the developed reaction system is its ability to enable the reaction under the mild conditions that constitute room temperature using photocatalysts that act under visible light (LED lamp irradiation). Fluoroalkyl groups, including trifluoromethyl groups, have a big influence on the chemical/metabolic stability and bond selectivity in pharmaceutical products, and are known to deliver improvements in drug activity, so the new reaction system is expected to see extensive use in the field of medicinal/agrochemical product development in the future.2
References
(1)R. Tomita, Y. Yasu, T. Koike, M. Akita, Angew. Chem. Int. Ed., 2014, 53, 7144-7148.
(2)Fluorine in Medicinal Chemistry and Chemical Biology (Eds: I. Ojima), Wiley-Blackwell, Chichester, 2009.



