Latest Research
- 2015.02.27
Fast proton conduction mechanism in highly concentrated acids -Packed acid mechanism
Polymer electrolyte fuel cells (PEFCs) have attracted much attention in recent years because of their high energy-conversion efficiency even at low temperatures. Although commercialization of PEFCs as automotive and residential applications has been started in Japan, further improvement in cost, performance and durability is still required.
One of the important issues to be solved in PEFCs is a rapid decrease in proton conductivity of standard electrolytes, perfluorosulfonated polymers, with decreasing relative humidity (RH), which hinders nonhumidified operation of PEFCs. On the other hand, PEFCs produce water by their reaction. Thus, the proton conductors must retain high proton conductivity over a broad RH range from low to high RH. Previous studies in our group revealed that high density of acid leads to a low activation energy of proton conduction.1)
In the present study, highly concentrated (packed) acid structure was formed at heterophase interfaces of inorganic and organic proton conductors as shown in Fig. 1. Inorganic proton conductors were highly dispersed in organic polymer electrolytes by utilizing capping phenomena, which is multipoint adsorption of polymers on surface-modified zirconia precursors. The precursors were then converted into proton conductors, zirconium sulphophenylphosphonate (ZrSPP). Polymer electrolytes used were sulfonated poly(arylene ether sulfone) (SPES).
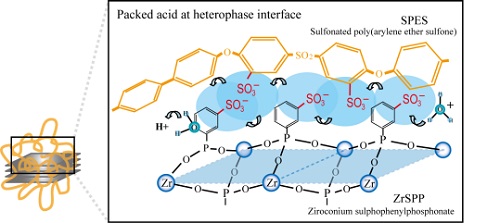
Fig. 1 Highly concentrated (packed) acid at heterophase interfaces of inorganic and organic proton conductors
TEM image analysis showed that ZrSPP was homogeneously dispersed in the composite of ZrSPP and SPES prepared by capping phenomena (capping-ZrSPP-SPES). In the FT-IR spectra of capping-ZrSPP-SPES, the peak of sulfonic acid, the O-S-O asymmetric stretching vibration, shifted to a higher wavenumber, suggesting the formation of packed acid structure. Fig. 2 shows the proton conductivities measured at 90oC. Conductivity of capping-ZrSPP-SPES was higher than the sum of the conductivities of each single material. These results suggests that packed acids are effective in increasing the proton conductivity.2)
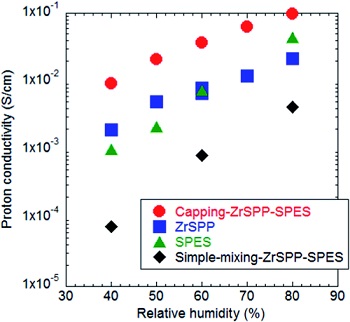
Fig. 2 Proton conductivities measured at 90oC 2)
The behavior of protons and water molecules were evaluated by 2H and 17O magic-angle spinning solid-state nuclear magnetic resonance (MAS NMR) spectroscopy at 47oC, -15oC, and -39oC. Fig. 3 shows NMR spectra of capping-ZrSPP-SPES. 2H-MAS NMR spectra show that protons in capping-ZrSPP-SPES retained their mobility at -39oC, even though the water molecule, a general carrier of protons, did not move as shown by 17O-MAS NMR spectra.2) This unusual proton behavior was not observed in other samples: simple-mixing-ZrSPP-SPES and SPES.3)
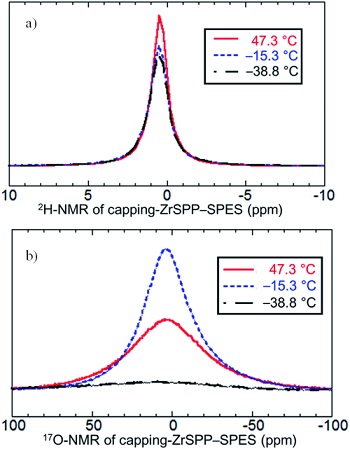
Fig. 3 a) 2H-MAS NMR and b) 17O-MAS NMR spectra
of capping-ZrSPP-SPES
Ab initio molecular dynamics calculations using models were then performed to explain these experimental results. In loosely associated acids, which represent the structure of conventional polymer electrolytes, protons are shuttled and trapped within one pair of proton donor and acceptor; this back-and-forth hopping is denoted as pseudo-shuttling. In packed acid structure, however, pseudo-shuttling is eliminated by H-bonds formed from other proton donors. Then, the protons can be reoriented to other proton acceptors, and thus successive proton conduction occurs as shown in Fig. 4. We denoted this proton conduction mechanismas "packed acid mechanism".
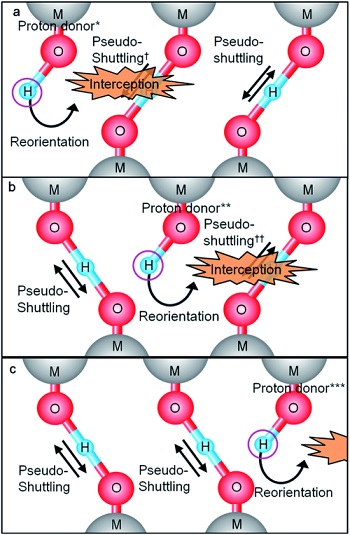
Fig. 4 Schematic illustration of packed acid mechanism 2)
Since the packed acid mechanism does not require water movement to conduct protons, the mechanism will be the key to achieve materials with low dependence of proton conductivity on RH.
1) N. Hara, H. Ohashi, T. Ito, T. Yamaguchi, J. Phys. Chem. B, 113, 4656-4663 (2009).
2) T. Ogawa, T. Aonuma, T. Tamaki, H. Ohashi, H. Ushiyama, K. Yamashita, T. Yamaguchi, Chem. Sci., 5, 4878-4887 (2014).
3) T. Ogawa, K. Kamiguchi, T. Tamaki, H. Imai, T. Yamaguchi, Anal. Chem., 86, 9362-9366 (2014).



