Latest Research
- 2015.12.01
Heme-Catalyzed Tyrosine Click Reaction with Luminol Derivatives
The chemical modification of proteins with synthetic probes is an important technique in chemical biology. It is one of the essential tools for the development of protein-based therapy and protein imaging technology.
To achieve reliable bioconjugation in the chemical modification of proteins, the following requirements should be met: (1) stable covalent bond should be formed, (2) specific moiety of a protein should be reacted chemoselectively, (3) reaction should proceed rapidly in aqueous solution, (4) conversion should be high under physiological pH and mild temperature.
In the attempt to modify native proteins, various bioorthogonal chemical reactions that enable irreversible bond formation in any of the natural amino acids have been developed. Much of the chemical modification of native proteins, however, depends on nucleophilic reagents, such as N-hydroxysuccinimide (NHS) ester and maleimide, and relies on the modification of lysine and cysteine residues. While those methods have significantly contributed to native protein modification, alternative methods that can modify different amino acid residues are still required.
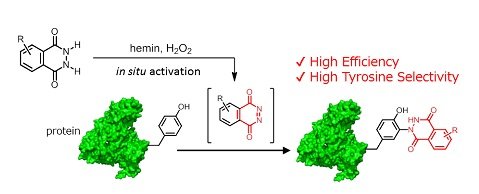
We developed a tyrosine-selective bioconjugation reaction1 using iron-porphyrin complex, hemin (heme B).2,3 Tyrosine-selective chemical modification was achieved using in situ hemin-activated luminol derivatives (Figure 1).
Figure 1. Tyrosine-selective chemical modification using luminol derivative.
As the previous tyrosine modification method, Barbas and co-workers reported a click-like ene-type reaction of tyrosine with a cyclic diazodicarboxyamide compound, 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD)4. Although PTAD derivatives are very promising for protein functionalization, they are unstable under physiological condition and generated isocyanate byproducts which are highly nucleophilic and react with amino groups, such as lysine residues and N-terminal amino groups. The relatively low selectivity for a tyrosine residue is caused by those side reactions. We thought that a reactive diazodicarboxyamide, which does not generate a nucleophilic byproduct, would enable us to modify the tyrosine residue selectively. We focused on the intermediate of the luminol reaction as a reactive diazodicarboxyamide. Luminol is known to induce the chemiluminescence in the presence of hemin and H2O2, however, we found that N-methylated luminol derivatives reacted with tyrosine residue and formed the covalent bond (8.55 M-1s-1) instead of chemiluminescence reaction under the same conditions. Tyrosine residues in peptide and protein were modified efficiently with N-methylated luminol derivatives through a single-electron oxidation mechanism in the presence of hemin and H2O2.
Tyrosine-specific chemical modification of the model protein revealed that the surface-exposed tyrosine residues were selectively linked with labeling reagent. We succeeded in the functionalization of several proteins using azide-conjugated labeling reagent and alkyne-conjugated fluorescent dye by copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) or copper-free click chemistry using dibenzocyclooctyne (DBCO).
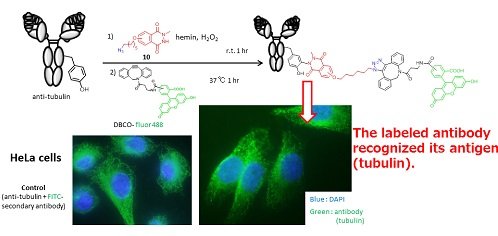
We succeeded in the modification of antibody (Figure 2). The recognition of antigen was retained after the modification, although the protein structures were possibly damaged by oxidation with H2O2 under the current reaction conditions. The current tyrosine-selective and high-efficiency reaction provides an attractive strategy for the modification of peptides and proteins, and a new methodology for protein modification and immobilization.
Figure 2. Antibody Modification with N-methylated luminol derivative.
In this method, luminol derivative labels only tyrosine residue efficiently. It does not react with other 19 kinds of natural amino acid residues and phosphorylated tyrosine residue. So, we thought this labeling reaction is applicable to the detection of phosphorylation state of peptide substrate of tyrosine kinase and tyrosine phosphatase. Applications to the screening assay of tyrosine kinase and tyrosine phosphatase are in progress.
References
1 McKay, C. S.; Finn, M. G. Chem. Biol. 2014, 21, 1075-1101.
2 Sato, S.; Nakamura, K.; Nakamura, H. ACS Chem. Biol. 2015, in press.
3 Shudofsky, A. D. Chem. Res. Toxicol. 2015, 28, 2086-2087. (spotlight feature of this work)
4 Ban, H.; Gavrilyuk, J.; Barbas, C. F. J. Am. Chem. Soc. 2010, 132, 1523-1525.