Latest Research
- 2014.05.08
Highly Selective Heterogeneous
Organic synthesis on Copper-planted Mesoporous Silica Materials
Introduction
Immobilization of a homogeneous catalyst onto solid support through pendant-type (linked) ligands is a powerful way to achieve advanced organic reactions in heterogeneous medium. In order to maintain catalytic activity as well as special characters, for example stereoselectivity, of the mother homogeneous catalyst, keeping a catalytic center away from a support had been usually thought to be desirable. On the other hand, our interest is focused on the use of active site on mesoporous silica materials and finding a specific property of the surface for selective organic reactions. Due to the following characteristic feature of mesoporous materials, our group believe that new reactivity of metal catalyst as well as modification with the appropriate additives will be possible to realize effective heterogeneous organic reaction.
1) Ordered nano-sized porous structure and uniform surface will be suitable for access of small organic molecules, and will provide a uniform transition state for their reactions.
2) Many kinds of metal species can be immobilized with highly dispersion state through template-ion exchange method. We additionally found that oxidation state as well as aggregation state of several metals can be controlled by designing of surface elements.
We will here introduce our efforts on heterogeneous selective organic reactions using Cu-planted MCM-41-type mesoporous silica materials as immobilized base-metal catalyst. As shown in the following topics, some of results evidence that copper, one of the typical base-metals can be an alternative element of precious metals such as rhodium and palladium which had played very important roles in conventional organic reactions (Fig. 1).
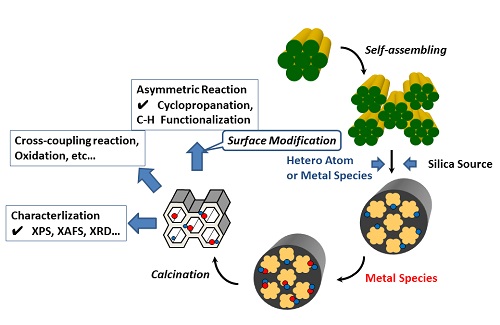
Fig. 1
Chemo- and/or Stereo-selective Reactions with Modifier/
Cu-MCM-41
Cyclopropanation of styrene derivatives with diazo esters is one of the classical asymmetric reactions catalyzed by chiral Cu complexes. On the other hand, very little is known concerning stereoselective versions with O¬-functionalized olefin substrate such as enol ethers. The BOX (L1) modified-Cu-MCM-41 was proven to be an effective asymmetric heterogeneous catalyst for such kinds of asymmetric reactions with bulky silyl enol ethers with diazo ester. Almost a single diastereomer of the corresponding product in the reaction of 1 with 2 was obtained in a good yield with an excellent enantioselectivity. The result is almost competitive to that of well-known chiral Rh dinuclear catalyst (Scheme 1).
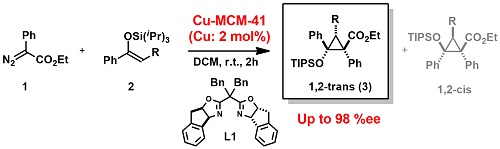
Scheme 1
The catalysis was next applied into asymmetric C-H bond functionalization of indole with diazo esters which gave chiral indole 3-acetic acids (typical structure of a plant hormone). The reactions of ethyl α-diazo β,γ-unsaturated carboxylates on the L2-modified Cu-MCM-41 proceeded and the corresponding α-(3-indolino)-β,γ-unsaturated ester 6 with good selectivity. The unexpected γ-regioisomer 8 could be selectively produced by switching chiral modifier from L2 to L1, and its enantioselectivity was reached up to 89 % ee (Scheme 2).
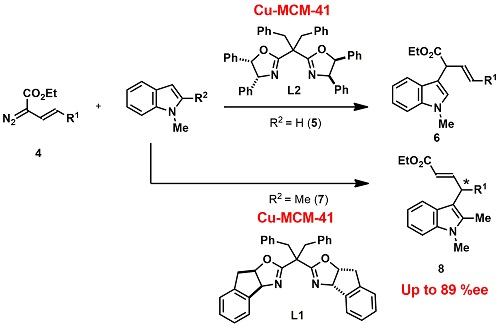
Scheme 2
C(sp2)-C(sp) and C(sp2)-C(sp2) Cross-Coupling Reactions on Cu-MCM-41
Sonogashira coupling of terminal alkynes and aryl halides is one of the most fundamental cross-coupling reactions which give disubstituted alkynes. Palladium catalyst usually shows high activity with the coexistence of copper, but more ecological and inexpensive process catalyzed solely by copper has attracted attention. The catalysis of our supported Cu was first proven in reactions of several iodoarenes with terminal alkynes in the presence of Cs2CO3 as a base (Figure 2). The control experiments using the parent MCM-41 and homogeneous Cu salts indicated no activity of MCM-41 itself or Cu alone.
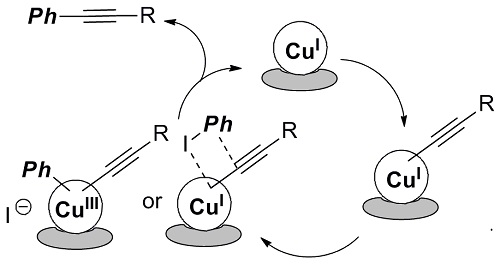
Fig.2
Cu-catalyzed arylation with aromatic compounds bearing amide group using hypervalent iodine is one of the promising methods for replacing conventional cross-coupling with organometallic reagents. The heterogeneous reaction of non-functionalized aromatic compounds such as m-xylene with Ph2IOTf in the presence of Cu-MCM-41 was found to proceed to afford the desired biaryl product. Reactions with amides provide m-phenylated biphenyl compounds selectively (Scheme 3). Those reactions will provide new opportunities for applying solid base-metal catalysis for cross-coupling reactions.
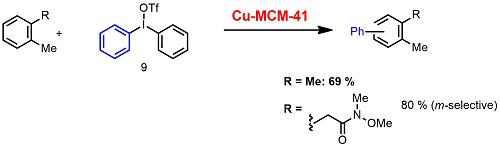
Scheme 3
Conclusion
Four kinds of selective organic reactions on copper-planted mesoporous silica catalyst was introduced. Our attention is now focused on exploring new chemical phenomenon taking place on the surface of porous materials. By using specific character of surface active sites, truly effective chemical transformation process will be created.




