Latest Research
- 2016.09.01
- Yamaguchi-Kuroki Group
Highly active and durable oxygen reduction reaction catalysts for polymer electrolyte fuel cells ~ Carbon-free, connected nanoparticle catalysts possessing a porous, hollow structure ~
Polymer electrolyte fuel cells (PEFCs) are promising energy devices for residential and automotive applications because of their high energy conversion efficiency, even at low temperatures. To make PEFCs more affordable and popular, reduction of cost and improvement of durability and are necessary, especially by improving the activity and durability of the cathode. Because of its low activity, a high platinum (Pt) loading (about 50 g Pt per vehicle) is required in conventional fuel cell vehicles. Conversely, conventional cars also use 3-5 g (estimated value) of Pt group metals to purify exhaust gases. Thus, a ten-fold increase in the activity of Pt or a reduction in the quantity of Pt to one tenth could solve the difficulties associated with Pt use in PEFCs. Another issue in PEFCs is degradation of carbon supports used in conventional catalysts (Fig. 1a). Carbon corrosion occurs when hydrogen and oxygen co-exist in the anode, which mainly occurs during start-stop operations. A possible way to intrinsically solve the problem of carbon corrosion is to employ an unsupported, carbon-free catalyst.
We employ connected Pt-Fe nanoparticle catalysts with a porous, hollow structure as a cathode catalyst in PEFCs for the first time (Fig. 1b). Because the fused Pt-Fe network structure is formed without sacrificing the high surface area of the nanoparticles and expected to conduct electrons through the capsules without any support, the catalysts can act as carbon-free electrocatalysts.
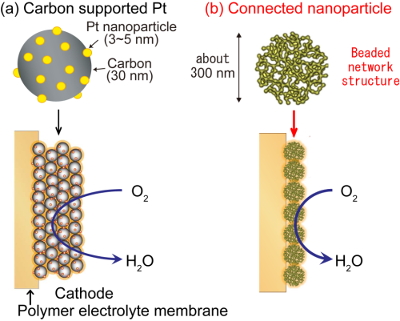
Fig. 1 Schematic illustration of cathode catalysts layers in polymer electrolyte fuel cells with (a) conventional carbon supported Pt catalysts and (b) Pt-Fe connected nanoparticle catalysts.
Connected Pt-Fe nanoparticle catalysts were synthesized via supercritical treatment of Pt-Fe nanoparticles formed on surface-modified silica particles, followed by dissolution of the silica particles in sodium hydroxide. Thermally fused Pt-Fe nanoparticles formed a Pt-Fe network structure with a pore size of ~10 nm and a crystallite size of approximately 6-7 nm (Fig. 2).
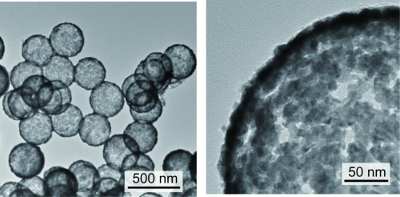
Fig. 2 TEM images of the connected Pt-Fe catalysts with porous hollow capsule structure.1)
The oxygen reduction reaction activity of the connected Pt-Fe catalysts measured in an acidic solution were 9 times that of the state-of-the-art commercial catalyst as shown in Fig. 3. The specific activity of the connected Pt-Fe catalysts was even higher than that of Pt-Fe/C, implying that the alloying with Fe cannot solely explain the reason for the improvement.1)
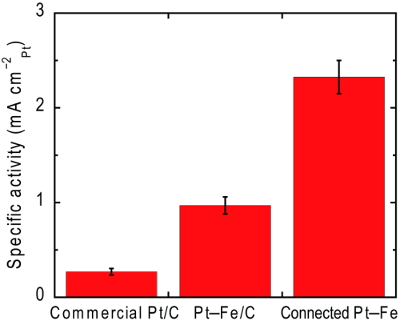
Fig. 3 Surface-area-specific activities for oxygen reduction reaction of the catalysts measured in 0.1 M HClO4.1)
Membrane electrode assembly (MEA) was then fabricated using the connected Pt-Fe catalysts as the cathode catalyst. Cross-sectional SEM images of the MEA (Fig. 4a) showed that carbon-free connected catalysts enabled the formation of a thin cathode with a thickness of 1-1.5 mm, nearly one-fifth that of a conventional electrode.1) Investigation on the relationship between the catalyst-layer nanostructures and the fuel-cell performances revealed that the uniform and thin ionomer coating produced a higher cell performance than the nonuniform and partially thick coating.2) Then, the durability of the connected Pt-Fe catalysts in the MEA was evaluated using a start-stop durability testing protocol, which mainly accelerates carbon corrosion. The elimination of the carbon support in the catalyst layer resulted in the high durability during the start-stop durability test, while conventional catalysts drastically degraded (Fig. 4b).1)
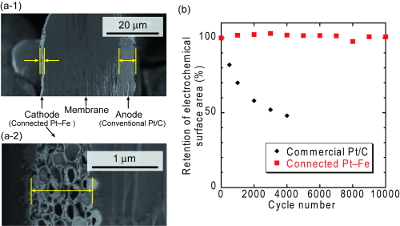
Fig. 4 (a) Cross-sectional SEM images of an MEA with a cathode composed of the connected Pt-Fe catalysts.
(b) Change in the electrochemical surface area of the catalysts during start-stop durability test using the MEA at 80 °C. 1)
The connected Pt-Fe nanoparticle catalysts with a beaded network structure were shown to be promising as a cathode catalyst in PEFCs because of their high activity and durability. Further investigation should be performed to clarify the reasons of the high activity of the connected nanoparticle catalysts, and to improve the cell performance by utilizing the merit of the thin electrode layer.
1) T. Tamaki,H. Kuroki, S. Ogura, T. Fuchigami, Y. Kitamoto, T. Yamaguchi, Energy Environ. Sci., 8, 3545-3549 (2015)
2) H. Kuroki, T. Tamaki, T. Yamaguchi, J. Electrochem. Soc., 163, F927-F932 (2016).



