Latest Research
- 2015.08.03
Development of a single component solution-processable thermally activated delayed fluorescence materials for organic light emitting diodes
OLEDs (Fig.1) are already in practical use; however, the device is still mainly fabricated by vacuum deposition of low molecular weight materials. For truly low cost and large area applicable fabrication process, the solution processability of the material is very important. Generally, for OLED materials, a uniform amorphous film is necessary. Therefore, polymeric materials were the main area of solution processable materials, but they suffer from a wide molecular weight distribution, inhomogeneity, low solubility, and impurities.
The development of emitting materials for OLEDs has started with fluorescence, moved to phosphorescence, and recently reached thermally activated delayed fluorescence (TADF).1TADF has the advantage of a high internal quantum efficiency (up to 100%) and low cost (rare metal free).2 However, to the best of our knowledge, a non-doped solution processable TADF material is still not developed. The important design principle of a TADF material is to spatially separate the HOMO and LUMO, because this reduces the difference in the singlet and triplet energy level (DES-T). Previously, we revealed that simple carbazole dendrimers3have an outer-layer electron rich potential gradient, i.e., a LUMO at the inner layer, and the HOMO at the outer-layer.4 The modification of an acceptor molecule to the core will extend this potential gradient and reduce the DES-T that leads to the TADF expression.
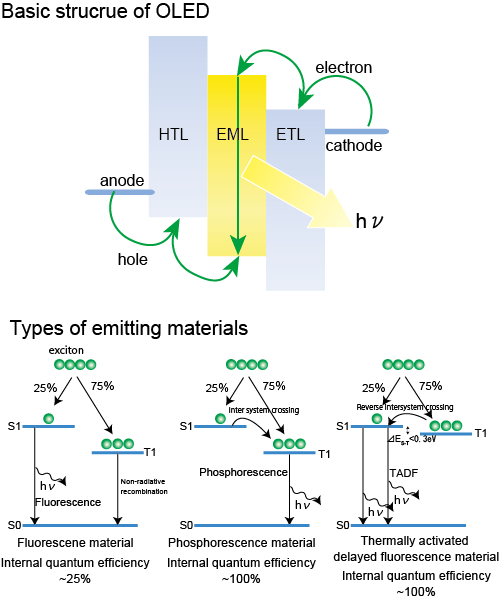 Fig.1 Basic structure of OLEDs, and three types of emitting materials.
Fig.1 Basic structure of OLEDs, and three types of emitting materials.
An s-triazine core carbazole dendrimers were synthesized (Fig.2a), and the luminescence properties were measured. The photoluminescence lifetime measurement of the neat film clearly showed the existence of long lifetime (ms order) component. This is typical behavior for TADF materials. OLED devices with a structure of ITO/PEDOT-PSS(30 nm)/dendrimer(35 nm)/TPBI(40 nm)/Ca(10 nm)/Al were fabricated (Fig.2 b,c). The dendrimers acted as the emitting layer. The maximum external quantum efficiency (EQE) of the OLED device reached 2.4% (G2TAZ), 3.4% (G3TAZ), and 1.5% (G4TAZ). There values are definitely higher than the EQE calculated with the assumption that the singlet states generated electrically, i.e. 25% of excitons, can contribute the emission. It is indicating that GnTAZ is harvesting the electrically-generated triplet excitons through TADF process. Thus, GnTAZ is the first solution-processable, single-component (non-doped), and high molecular weight TADF material for OLEDs(Fig.3).5
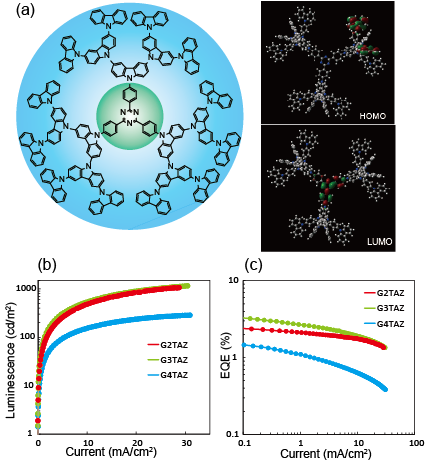
Fig. 2 a) Structure, HOMO, and LUMO orbital of triazine core carbazole dendrimer (G3TAZ). b) Dependence of the luminescence on the current density. c) Dependence of the external quantum efficiency on the current density. The OLED devices structure for b), and c) was ITO/PEDOT:PSS(30 nm)/GnTAZ(35 nm)/TPBI(40 nm)/Ca(10 nm)/Al.
Fig.3 Photo of an OLED device with G2TAZ as the emitting layer.
This work was supported in part by the Nano-Macro Materials, Devices, and System Research Alliance Project,
1 A. Endo, M. Ogasawara, A. Takahashi, D. Yokoyama, Y. Kato, C. Adachi, Adv. Mater., 2009, 21, 4802-4906.
2 H. Uoyama, K. Goushi, K. Shizu, H. Nomura, C. Adachi, Nature, 2012, 492, 234 - 238.
3 a) A. Kimoto, J.-S. Cho, M. Higuchi, K. Yamamoto, Macromolecules, 2004, 37, 5531-5537. b) A. Kimoto, J.-S. Cho, K. Ito, D. Aoki, T. Miyake, K. Yamamoto, Macromol. Rapid Commun. 2005, 26, 597-601. c) K. Albrecht, Y. Kasai, A. Kimoto, K. Yamamoto, Macromolecules, 2008, 41, 3793-3800.
4 K. Albrecht, K. Yamamoto, J. Am. Chem. Soc. 2009, 131, 2244-2251.
5 K. Albrecht, K. Matsuoka, K. Fujita, K. Yamamoto, Angew. Chem. Int. Ed. 2015, 54, 5677-5682.



