Latest Research
- 2015.04.08
Organic structure-directing agent free synthesis of CHA-type aluminosilicate zeolite
Microporous crystalline zeolites have been utilized in many industrial technologies, including gas adsorption, ion exchange, separation and catalysis for their unique porosity and high surface area. Until now, more than 200 different framework topological structures have been known [1]; however, only some of which have important commercial values. Nevertheless, zeolites are still attracting a wide research interest because novel frameworks may have new physicochemical properties and potential applications. So far, a variety of successful methods and strategies have been employed to synthesize zeolites with new topological structures, summarized in excellent papers.
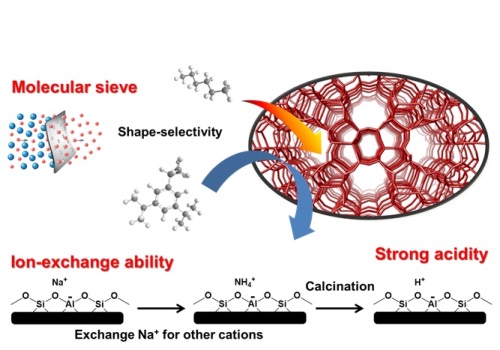
Fig. 1 Properties of zeolite.
Recently, 8-membered ring (8MR) zeolites and zeotype materials have attracted much attention in expectation of selective catalysis due to their small pores. For example, CHA-zeotype materials such as SSZ-13 and SAPO-34 show a remarkable catalytic activity for methanol to olefins (MTO) reaction to provide ethylene and propylene, which are important chemicals for the polymer industry.
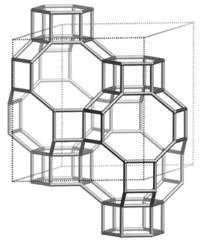
Fig. 2 Structure of CHA-type zeolite
[Al]-SSZ-13, the high-silica CHA-type aluminosilicate zeolite, is directly synthesized from an amorphous aluminosilicate gel by using N,N,N-trimethyladamantammonium hydroxide (TMAdaOH) as an organic-structure-directing agent (OSDA) [2]. However, from the viewpoint of the development of simple, economical and environmentally benign processes for zeolite synthesis, the method for the OSDA-saving or -free synthesis of the zeolite has been highly desirable. In preparing the CHA-type aluminosilicate zeolite, the reduction in the amount of TMAdaOH and the use of inexpensive OSDAs such as benzyltrimethylammonium hydroxide have been extensively investigated [3-5].
By contrast, the CHA-type aluminosilicate zeolite is formed in nature as "chabazite" mineral, and it has been easily synthesized through the interzeolite conversion of the FAU zeolite without using OSDAs [6]. However, the obtained CHA-type zeolite has a high Al content; the Si/Al atomic ratio is below 3. Our target in this study is to develop the direct synthesis method of the CHA-type aluminosilicate zeolite from an amorphous aluminosilicate gel without using any OSDAs to control the Si/Al ratio in the product.
Since the seed-assisted OSDA-free synthesis of Beta zeolite reported by Xiao and his co-workers [7], OSDA-free zeolite synthesis has been extensively studied because such approach can not only decrease the production steps and costs but also contribute to environmentally benign synthesis of advanced materials [8, 9]. We have also established the synthesis route for the seed-assisted OSDA-free synthesis of RTH-type zeolite [10]. Here, we report the direct crystallization of the CHA-type aluminosilicate zeolite in the presence of seed without using OSDAs and its application as catalyst for the MTO reaction [11]. Thus prepared zeolite was designated as "OSDA-free [Al]-CHA".
For typical synthesis, sodium-type [Al]-SSZ-13 (Si/Al = 7.7) for seed crystals was synthesized by using TMAdaOH as OSDA and calcined at 600 ºC for 10 h to remove the OSDA. In the synthesis of CHA-type aluminosilicate zeolites in the absence of OSDA, sodium alminate was added to an aqueous solution containing NaOH and KOH. A silica source was added into the mixture. The calcined [Al]-SSZ-13 (20 wt% of added silica source) was added to the mixture as seed. The resultant mixture was hydrothermally treated at 170 ºC for 1 day with tumbling at 20 rpm. For the purpose of varying the Si/Al ratio, CsOH instead of KOH was used. Fig. 3 shows the synthesis procedures of [Al]-SSZ-13 and OSDA-free [Al]-CHA.
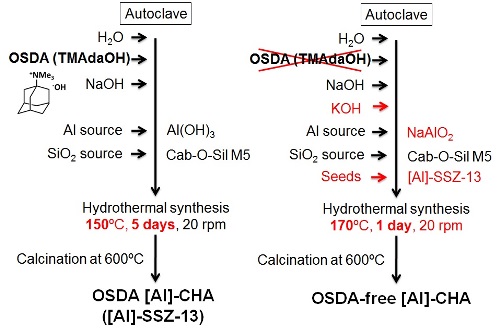
Fig. 3 Synthesis procedures of [Al]-SSZ-13 and OSDA-free [Al]-CHA.
Fig. 4 shows XRD patterns of calcined [Al]-SSZ-13 and the products synthesized with or without seed crystals in the absence of OSDAs. The product synthesized with calcined [Al]-SSZ-13 as a seed in the absence of OSDAs exhibited the typical diffraction pattern of CHA-type zeolite, although the product had a low intensity of the peaks of the pattern in comparison with calcined [Al]-SSZ-13. Note that the yield of the product was higher (ca. 51%) than that of the seed crystals added, indicating that the amorphous aluminosilicate gel was used for the crystallization of the CHA zeolite. As a control, the hydrothermal treatment of the starting gel without adding seed crystals led to the GIS-type zeolite, indicating that the seed crystals of CHA-type zeolite in the starting gel are inevitable for the formation of CHA-type zeolite. The Si/Al ratio of the OSDA-free CHA zeolite calculated from the spectrum was 3.2.
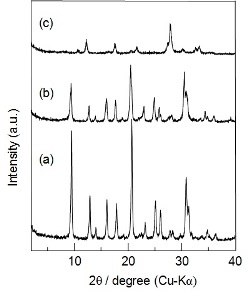 Fig. 4 XRD patterns of (a) [Al]-SSZ-13 and the products synthesized (b) with the calcined [Al]-SSZ-13 as a seed (OSDA-free [Al]-CHA) and (c) without seeds in the absence of OSDA, respectively.
Fig. 4 XRD patterns of (a) [Al]-SSZ-13 and the products synthesized (b) with the calcined [Al]-SSZ-13 as a seed (OSDA-free [Al]-CHA) and (c) without seeds in the absence of OSDA, respectively.
In order to investigate the effect of the seed crystals on the formation of the CHA phase, the hydrothermal treatment was carried out by varying the seed content (Fig. 5). The hydrothermal treatment of the amorphous gel without any seed crystals resulted in the formation of the PHI phase with a yield of about 30% (Fig. 5a). Interestingly, by adding 2 wt% of the seed crystals, the peaks attributed to the CHA zeolite was slightly observed (Fig. 5b). The peak intensities were not significantly increased and the yield of the product was unchanged when the amount of the seed crystals was increased from 2 to 10 wt% (Figs. 5b-d). However, when its amount was increased up to 20 wt%, the CHA zeolite with high crystallinity was successfully synthesized (Fig. 5e). Namely, the addition of the seed crystals with the CHA topology is essential to the formation of the CHA phase from the amorphous aluminosilicate gel in the absence of OSDAs, and the crystallization rate depends on its content.
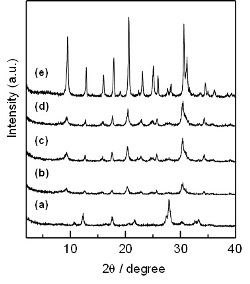 Fig. 5 XRD patterns of the products synthesized by using calcined [Al]-SSZ-13 (seed) of (a) 0 wt% (yield: 31%), (b) 2 wt% (yield: 32%), (c) 5 wt% (yield: 31%), (d) 10 wt% (yield: 28%), and (e) 20 wt% (yield: 36%) as a seed in the absence of OSDAs.
Fig. 5 XRD patterns of the products synthesized by using calcined [Al]-SSZ-13 (seed) of (a) 0 wt% (yield: 31%), (b) 2 wt% (yield: 32%), (c) 5 wt% (yield: 31%), (d) 10 wt% (yield: 28%), and (e) 20 wt% (yield: 36%) as a seed in the absence of OSDAs.
The crystallization process of the CHA phase was investigated through the hydrothermal treatments of the amorphous aluminosilicate gel containing the seed with the reaction times varied. Fig. 6 shows the evolution of XRD patterns of the products synthesized in the absence of OSDAs with the crystallization time varied. The peaks derived from the seed were clearly observed in the XRD pattern of a mixture of 20 wt% seed crystals and fumed silica. After 1 h of the hydrothermal treatment, no peak was observed, indicating that the seed crystals were mostly dissolved during the hydrothermal treatment in the highly alkaline gel. After 3 h, the peaks attributed to only the CHA structure appeared, and the peak intensities were gradually increased along with the treatment time. No further change in the peak intensity was observed after 24 h. When the treatment time was further prolonged to 40 h, the peaks attributed to the MOR structure appeared at the expense of those to the CHA structure.
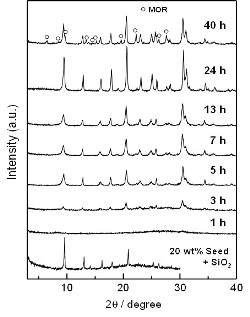
Fig. 6 XRD patterns of the products synthesized in the absence of OSDAs with the crystallization time varied.
Fig. 7 shows the SEM images of the products obtained through the hydrothermal treatment for different times. After 1 h of the treatment, the cubic crystals of [Al]-SSZ-13 were no longer observed, being consistent with the XRD findings (Fig. 4). The growth of particles was observed after 3 h. When the starting gel was hydrothermally treated for 7 h, the particles were agglomerated to form large discoid masses 2 μm in size. Further increase in the treatment time to 24 h resulted in the formation of the agglomeration of roundish cubic crystals 0.07 - 0.15 μm in size, whose shape was similar to that of the synthetic chabazite. These findings are different from the typical OSDA-free synthesis of zeolites where the particle size of a target zeolite is usually larger than that of seed crystals.
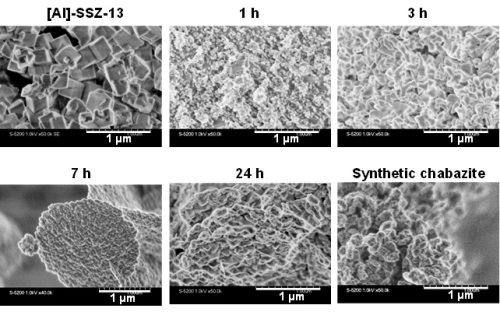
Fig. 7 FE-SEM images of the products synthesized in the absence of OSDAs with the crystallization time varied.
In order to further decrease the Al content introduced into the framework, CsOH was employed in place of KOH. Since CsOH has higher alkalinity than KOH, the added amount of alkali cations may be decreased with the alkalinity in the amorphous gel kept. Moreover, the introduction of cesium cations into the cages of the CHA zeolite is limited due to their large cation sizes. Hence, the effect of alkali cations were investigated by using Cs+, which is larger than K+, in combination with Na+. Consequently, the Si/Al ratio can be increased to 5.2 with the crystallinity and the yield of the product kept when CsOH was used in place of KOH.
Finally, acid properties of OSDA-free CHA-type aluminosilicate zeolites and their catalytic performances in the Methanol-to-Olefins (MTO) reaction were investigated in details. The high-silica sample (Si/Al = 5.2) exhibited a longer catalytic life compared to the low-silica sample (Si/Al = 3.2), while OSDA-free CHA-type aluminosilicate zeolites were inferior to [Al]-SSZ-13 (Si/Al = 7.7).
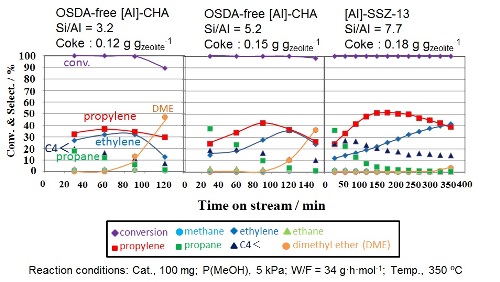
Fig. 8 MTO reactions over [Al]-SSZ-13 and OSDA-free [Al]-CHA catalysts.
In conclusion, pure CHA-type aluminosilicate zeolite with high crystallinity has been successfully synthesized from an amorphous aluminosilicate gel by a judicious use of alkali cations together with the addition of seed crystals with the CHA topology in the absence of organic-structure-directing agents (OSDAs). It was found that the crystallization time was shortened to 1 day by the seed-assisted method in comparison with 5 days of the crystallization through the conversion of FAU zeolite. The addition of the seed to the amorphous gel was essential to the synthesis of the CHA zeolite in the absence of OSDAs. A combination of alkali cations also played an important role in the crystallization of the CHA zeolite. The judicious use of alkali cations, CsOH together with NaOH, resulted in the increase in the Si/Al ratio of the CHA zeolite since the added total amount of alkali cations were able to be decreased.
[1] International Zeolite Association (URL: http://www.iza-structure.org/)
[2] S.I. Zones, U.S. Patent 4 544 538 (1985).
[3] S.I. Zones, R.A. van Nordstand, Zeolites 8 (1988) 166-174.
[4] S.I. Zones, J. Chem. Soc., Faraday Trans. 87 (1991) 3709-3716.
[5] M. Itakura, T. Inoue, A. Takahashi, T. Fujitani, Y. Oumi, T. Sano, Chem. Lett. 37 (2008) 908-909.
[6] H. Robson, Verified Synthesis of Zeolitic Materials, 2nd ed., Elsevier, Amsterdam, 2001, p. 123.
[7] B. Xie, J. Song, L. Ren, J. Li, F.-S. Xiao, Chem. Mater., 20 (2008) 4533-4535.
[8] Y. Kamimura, S. Tanahashi, K. Itabashi, A. Sugawara, T. Wakihara, A. Shimojima, T. Okubo, J. Phys.Chem. C, 115 (2011) 744-750.
[9] K. Itabashi, Y. Kamimura, K. Iyoki, A. Shimojima, T. Okubo, J. Am. Chem. Soc. 134 (2012) 11542-11549.
[10] T. Yokoi, M. Yoshioka, H. Imai, T. Tatsumi, Angew. Chem. Int. Ed, 48 (2009) 9884-9887.



