Latest Research
- 2014.11.24
Polymerization and copolymerization of ethylene and olefins by double-decker type dinuclear complexes
Polymerization of ethylene and α-olefins has been the important topics for academic research as well as industrial application. In addition to mononuclear complex catalysts, which include only one metal center (Fig. 1A), dinuclear complexes, which include two metal centers, have attracted recent attention as the catalysts for the polymerization. There have been the reports that some dinuclear complex catalysts actually show higher catalytic activity in ethylene/olefin polymerization and/or afford higher molecular weight polymers than the corresponding mononuclear complex catalysts. However, most of the conventional dinuclear complex catalysts bind the two complex units with a flexible tether (Fig. 1B). The cooperation between the two metal centers during the polymerization has remained unclear due to the flexible structure and there have been no example of the polymerization that is enabled only by using the dinuclear complex.
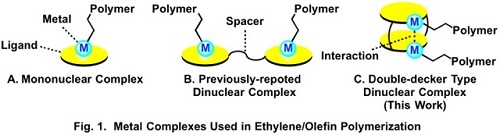
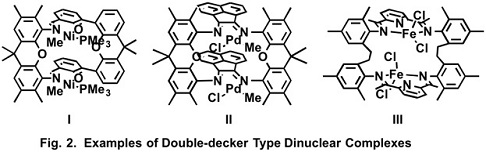
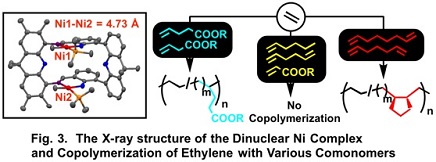
We designed double-decker type dinuclear complexes, which connect two mononuclear complexes with two spacers (Fig. 1C). The two metal centers are located in close position in the dinuclear complexes and we expected more effective cooperation between the two metal centers. Three types of double-decker type dinuclear complexes were synthesized (Fig. 2). X-ray crystallography of the dinuclear nickel complex (I) revealed the Ni-Ni distance of 4.73 Å, which is the shortest among the previously-reported similar dinuclear Ni complexes (Fig. 3).
The dinuclear Ni complex (I) shows higher activity for the ethylene polymerization than the corresponding mononuclear analogue and affords polyethylene with higher molecular weight.1) The copolymerization of ethylene with 1,6-heptadiene or 1,7-octadiene by the catalyst affords copolymer with the cyclic repeating units formed by cyclization of the dienes (Fig. 3). The ethylene-1,7-octadiene copolymer is suggested to adopt ladder structure. On the other hand, copolymerization of ethylene with 1,5-hexadiene or 1-hexene by the dinuclear complex or copolymerization of ethylene with non-conjugated dienes by the mononuclear complex does not proceed. Appropriate combination of the dinuclear complex and non-conjugated dienes is of importance for the efficient incorporation of the comonomer, which suggests diene coordinate to the two metal centers. The dinuclear complex is also effective for the copolymerization of ethylene with butenoate or pentenoate.
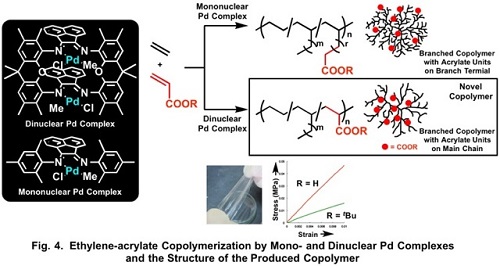
Ethylene-acrylate copolymerization catalyzed by mononuclear diimine Pd complex affords branched copolymer with acrylate units locating terminal of the branches. The copolymerization catalyzed by the dinuclear Pd complex (II), on the other hand, gives a copolymer with acrylate units on polymer main chain (Fig. 4).2) Such copolymer is unprecedented and firstly achieved by using the dinuclear complex. The copolymer obtained by the dinuclear Pd complex forms film, which is in contrast to that the copolymer obtained by the mononuclear complex is amorphous and oil at room temperature.
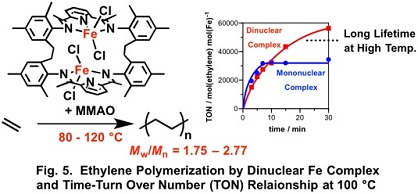
The dinuclear iron complex (III) is active for ethylene polymerization at 80 - 120 °C, whereas the corresponding mononuclear complex is deactivated at the temperature within10 minutes (Fig. 5).3) Similar high thermal stability of the dinuclear complexes are also observed in the polymerization by the dinuclear nickel and palladium complexes.
1) D. Takeuchi, Y. Chiba, S. Takano, K. Osakada, Angew. Chem. Int. Ed. 2013, 52, 12536-12540.
2) S. Takano, D. Takeuchi, K. Osakada, N. Akamatsu, A. Shishido, Angew. Chem. Int. Ed. 2014, 53, 9246-9250.
3) D. Takeuchi, S. Takano, Y. Takeuchi, K.Osakada, Organometallics 2014, 33, 5316-5323.




