Latest Research
- 2017.01.05
Development of the CON-type Aluminosilicate Zeolite with Unique Catalytic Performance in the MTO Reaction
1. Introduction
The Methanol to Olefins (MTO) reaction becomes highly important for producing lower olefins as important basic chemicals for the polymer industry instead of steam cracking of naphtha.1, 2 Various zeolites have been examined for the MTO reaction; there are numerous reports on the modifications of catalysts to promote the transformation of methanol to olefins and to control the selectivity to the desired products.3-13 To date, ZSM-5, SAPO-34 and SSZ-13 have been well known as excellent catalysts for the MTO reaction.3, 4
Nowadays due to the augmentation of production of ethene from ethane in the Middle East, fundamental chemical feedstock required by chemical industry is shifting from C2-C3 to C3-C4 olefins. The development of the zeolite catalysts that can selectively produce C3-C4 olefins by the conversion of methanol has been strongly desired. Since zeolites having small-pore do not meet this demand, we have adopted aluminosilicate zeolite having 12MR channels as catalysts for the MTO reaction to achieve a high selectivity to C3-C4 olefins and a long catalytic life. Actually, faujasite (FAU), beta (*BEA) and MCM-68 (MSE) zeolites were examined the catalytic activity in the MTO reaction.14 However, the CON-type zeolite, which consists of 3-dimensional channel system with 12-, 12- and 10-MR pores, as catalyst for the MTO reaction has attracted little attention to date. We have discovered a unique catalytic performance of the CON-type zeolites in the MTO reaction.
In 1993, SSZ-26 and SSZ-33, which are high-silica molecular sieves containing a three-dimensional pore system comprised of intersecting 10- and 12-ring pores, have been developed (Figure 1).15 They are a family of the CON-type zeolite, but their structures consist of the stacking of polymorphs A and B.16 The first report on the synthesis of the zeolite with a pure CON phase is a borosilicate "CIT-1" (designated as [B]-CON) developed in 1995.17 The CON-type aluminosilicate zeolite has also been synthesized by the post-synthesis method using deboronated [B]-CON.17, 18 The CON-type zeolite has been expected to show remarkable properties in the fields of catalysis and adsorption because of its unique structure with high accessibility and diffusibility; the 12- and 10-MR channels connect to form a large void at the intersections, providing access to the crystal interior through two pores.18, 19 Despite the attractive capabilities of the CON-type zeolites, a route for directly preparing CON-type aluminosilicate zeolites has not been developed.
We have developed the method for directly synthesizing Al-containing CON-type borosilicate zeolites, which are designed as [Al,B]-CON-D. They have proved to give an excellent catalytic performance in the MTO reaction. The differences in the acidic properties and catalytic performance between the directly- and post-synthesized CON-type aluminosilicate zeolites have also been clarified.
2. Direct-synthesis of the CON-type aluminosilicate zeolite
N,N,N-trimethyl-(-)-cis-myrtanylammonium hydroxide (TMMAOH) was used as an organic structure-directing agent (OSDA) for crystallizing the CON-type zeolite. The product was designated as "[Al,B]-CON-D (109, static)" when the zeolite was directly synthesized under the static conditions (the number in parentheses is the Si/Al atomic ratio in the product). The product was designated as "[Al]-CON-P" when the CON-type aluminosilicate zeolite was synthesized by the post-synthesis method.
First, we attempted to directly incorporate Al atoms into the CON framework in the absence of boron atoms ([Al]-CON-D); aluminum sulfate as Al source was introduced into the mother gel in place of boric acid, and the hydrothermal treatment was conducted under the same conditions as [B]-CON, which can be obtained after the hydrothermal treatment of the mother gel with the Si/B atomic ratio of 25 under the static conditions.17
At the atomic ratio of Si/B = 25 in the mother gel, the synthesis of [Al, B]-CON-D was conducted with the Al content varied (Figure 2). The mother gels with the Si/Al ratio below 100 did not give a pure CON-phase. However, when the Si/Al ratio in the mother gel was increased to 100, the CON-type zeolite with the Si/Al ratio of 117 was purely obtained after 21 days. The mother gels with the Si/Al ratios of 150 and 200 also led to the formation of the CON-type zeolite.
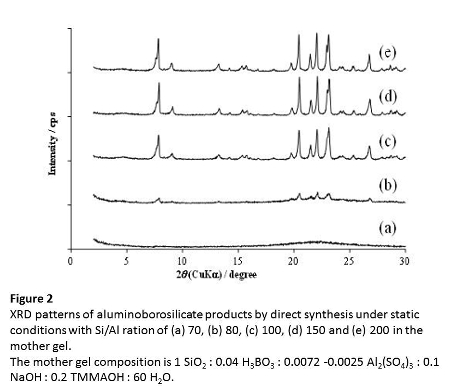
The synthesis conditions for the CON-type zeolites were investigated. We have also found that [Al,B]-CON-D can be synthesized in a shorter period and at a lower temperature by carrying out the hydrothermal treatment under the tumbling conditions (40 rpm); the mother gels with the Si/Al ratio ranging from 100 to 200 were purely crystallized into the CON-structure at 170 oC after 16 days. We also found that the tumbling condition led to the reduction in the particle size. Further investigations on the effect of the temperature of the hydrothermal treatment are currently under way. The crystallization of the CON-type zeolite in the absence of B was still unsuccessful under the tumbling conditions at 170 oC.
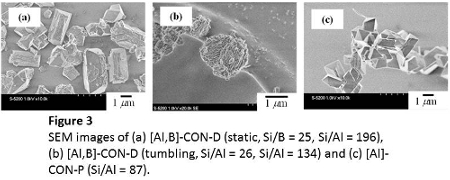
The FE-SEM images revealed that crystals of acuate-square prisms 2-4 μm in length were present in the [Al,B]-CON-D samples synthesized under static conditions, while [B]-CON consisted of acuate-square prisms 1-2 μm in length (Figure 3). The morphology of [Al,B]-CON-D synthesized under tumbling conditions was slightly different from that of the samples synthesized under the static conditions; a stack of smaller-sized particles was observed in the crystals of acuate-square prisms.
3. Catalytic performance of the CON-type aluminosilicate zeolites in the MTO reaction.
Thus prepared CON-type aluminosilicate zeolites were used as catalyst for the MTO reaction. Figure 4 shows the change in the conversion of methanol and the product selectivity along with time on stream (TOS) at 500 oC. For the CON-type zeolites catalysts, at the initial stage (time =10 min), the conversion of methanol was ca. 100%, the main product was propene and its selectivity reached to 50 %. Butenes were formed with the selectivity of about 20 %; among butenes, the selectivities to isobutene, 1-butene and trans-2- and cis-2-butenes were about 10, 25, 40 and 25 %, respectively. Note that the selectivity to ethene was as low as 5%.
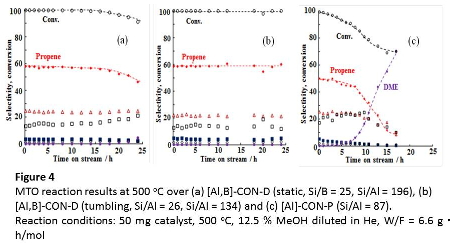
Interestingly, the directly synthesized CON-type aluminosilicate zeolites showed a much longer catalytic life than the zeolite synthesized by the post-synthesis method. For [Al]-CON-P (87), although the conversion of methanol and the selectivity to propene were 99 and 50% at the initial stage, respectively, these values were remarkably decreased to 81 and 35 %, respectively at TOS of 10 h. Instead of the decrease in the selectivity to propene, dimethyl ether (DME) was rapidly increased at TOS of 7 h. On the other hand, [Al,B]-CON-D (109, static) gave the 100 % conversion of methanol and the 60 % selectivity to propene even at TOS of 10 h. Butenes were constantly formed with the selectivity of about 20 % for 16 h. The selectivity to ethene was hardly changed; it was kept less than 5 % for 16 h. For a higher-silica type sample, [Al,B]-CON-D (196, static), the 100 % conversion was kept for 16 h with the products selectivities unchanged.
The very low selectivity to ethene on the CON-type aluminosilicate zeolites might be accounted for as follows. Based on the "dual-cycle concept" proposed by Olsbye and her co-workers1, 20 for the conversion of methanol, the low acid strength of the CON-type zeolites contributes to the suppression of aromatization of higher alkenes formed in the alkene methylation/cracking cycle. They also proposed that ethene is formed from the "aromatics-based cycle". Therefore, the use of the CON-type zeolites would result in lessened formation of ethene. The 3-dimensional large pores system with a high diffusibility would also suppress sequential reactions, oligomerization and the following cracking, in the pores, leading to the low selectivity to ethene. More interestingly, the CON-type zeolite synthesized under tumbling conditions ([Al,B]-CON-D (134, tumbling)) exhibited a much longer catalytic life than those synthesized under static conditions; the 100 % conversion was kept for 24 h with the selectivity to propene of about 60 % unchanged and the total selectivity for C3-C4 olefins was kept at about 80 %. The differences between the static and tumbling conditions in the direct synthesis were crystallization time, particle morphology and Al distribution in the framework. We have considered that, in addition to particle morphology, the Al distribution in the framework strongly affected the catalytic performance.
4. Difference in the Al distribution between the directly and post-synthesized CON-type zeolites.
Figure 5 shows the 27Al MAS MNR spectra of the products. A strong and broad peak at 54-59 ppm assigned to tetrahedrally-coordinated Al species irrespective of the preparation routes. Except for [Al]-CON-P (87), a small peak at -0.6 ppm assigned to octahedrally-coordinated Al species were also observed. Note that, for the directly synthesized CON-type zeolites, the main peak clearly consists of two peaks; the major peak appeared at 58 ppm, and a shoulder peak at 55 ppm. The shoulder peak at 55 ppm in the 27Al MAS NMR spectra might be assigned to tetrahedrally-coordinated Al species in the framework and/or extra-framework. These findings suggest that there is a significant difference in the environment of Al species, e.g., the location of Al atom in the framework, between the directly- and post-synthesized CON-type zeolites.
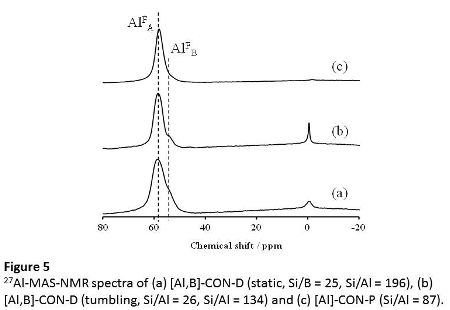
The 27Al MAS NMR spectra indicate that the CON-type zeolite has at least two kinds of different Al species. To clarify the difference in the Al distribution between the directly- and post-synthesized CON-type zeolites, the high resolution 27Al 3Q MQMAS NMR spectra were measured (Figure 6). In the 27Al MQMAS NMR spectra, the spectra of the samples directly prepared under the static conditions clearly showed two cross-sections, designated as "a" and "b". For the sample directly prepared under the tumbling conditions, [Al,B]-CON-D (134, tumbling), in addition to "a" and "b", the cross-section "c" was newly observed. Note that only one cross-section "a" was observed in the spectrum of [Al]-CON-P (87).
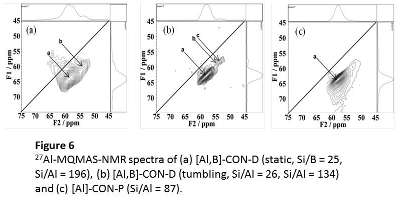
These findings indicate that there is a marked difference in the state of tetrahedrally-coordinated Al species in the framework between the directly and post-synthesized zeolites, and that there is also a significant difference in the Al distribution among the samples directly prepared under the static and tumbling conditions. We consider that the framework Al atoms corresponding to the cross-sections "b" and "c" contribute to the suppression of the formations of aromatics and heavy hydrocarbons. The presence of the framework Al atom "c" and small particle could explain the reason for the long catalytic life of [Al,B]-CON-D (134, tumbling) compared with [Al,B]-CON-D synthesized under static conditions.
5. Conclusions
In conclusion, the CON-type aluminosilicate zeolite was successfully synthesized by the direct-synthesis methods as well as the post-synthesis ones. The 27Al 3Q MQMAS measurements revealed that the preparation method have a marked influence on the Al distribution in the CON framework. We found a unique catalytic performance of the CON-type zeolites in the MTO reaction. In particular, the CON-type aluminosilicate zeolites directly prepared under the tumbling conditions exhibited a high propene selectivity, low ethene selectivity and a very long catalytic life compared to Beta and ZSM-5, and the total selectivity for C3-C4 olefins of 80 % was achieved. In addition to Lewis acid and defect sites and particle morphology, we have considered that the Al distribution in the CON framework also have a significant influence on the catalytic performance. Our findings will contribute to the development of the zeolite catalysts with acid sites in the pores properly controlled, and would broaden catalytic applications of the CON-type zeolites. This work was partially supported by Japan Technological Research Association of Artificial Photosynthetic Chemical Process (ARPChem) as shown in Figure 7.
REFERENCES
(1) Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T. V. W.; Joensen, F.; Bordia, S.; Lillerud, K. P. Angew. Chem. Int. Ed. 2012, 51, 5810-5831.
(2) Plotkin, J. S. Catal. Today 2005, 106, 10-14.
(3) Chen, J. Q.; Bozzano, A.; Glover, B.; Fuglerud, T.; Kvisle, S. Catal. Today 2005, 106, 103-107.
(4) Chang, C. D.; Chu, C. T.-W.; Socha, R. F. J. Catal. 1984, 86, 289-296.
(5) Zhu, Q. ; Kondo, J. N. ; Tatsumi, T.; Inagaki, S. ; Ohnuma, R.; Kubota, Y. ; Shimodaira, Y.; Kobayashi, H.; Domen, K. J. Phys. Chem. C 2007 111, 5409-5415.
(6) Zhu, Q.; Kondo, J. N.; Ohnuma, R.; Kubota, Y.; Yamaguchi, M.; Tatsumi, T. Micropor. Mesopor. Mater. 2008, 112, 153-161.
(7) Zhu, Q.; Hinode, M.; Yokoi, T.; Kondo, J. N.; Kubota, Y.; Tastumi, T. Micropor. Mesopor. Mater. 2008, 116, 253-257.
(8) Park, J. W.; Lee, J. Y.; Kim, K. S.; Hong, S. B.; Seo, G. Appl. Catal. A: General 2008, 339, 36-44.
(9) Zhu, Q.; Hinode, M.; Yokoi, T.; Yoshioka, M.; Kondo, J. N.; Tatsumi, T. Catal. Commun. 2009, 10, 447-450.
(10) Yokoi, T.; Yoshioka, M.; Imai, H.; Tasumi, T. Angew. Chem. Int. Ed. 2009, 48, 9884-9887.
(11) Kumita, Y.; Gascon, J.; Stavitski, E.; Moulijn, J. A.; Kapteijn, F. Appl. Catal. A: General 2011, 391, 234-243.
(12) Bleken, F.; Skistad, W.; Barbera, K.; Kustova, M.; Bordiga, S.; Beato, P.; Lillerud, K. P.; Svelle, S.; Olsbye, U. Phys. Chem. Chem. Phys. 2011, 13, 2539-2549.
(13) Wang, Q.; Cui, Z.-M.; Cao, C.-Y.; Song, W.G. J. Phys. Chem. C 2011, 115, 24987-24992.
(14) Dorset, D. L.; Weston, S. C.; Dhingra, S. S. J. Phys. Chem. B 2006, 110, 2045-2050.
(15) Lobo, R. F.; Pan, M.; Chan, I.; Li, H. X.; Medrud, R. C.; Zones, S. I.; Crozier, P. A.; Davis, M. E. Science 1993, 262, 1543-1546.
(16) Lobo, R. F.; Pan, M.; Chan, I.; Medrud, R. C.; Zones, S. I.; Crozier, P. A.; Davis, M. E. J. Phys. Chem. 1994, 98, 12040-12052.
(17) Lobo, R. F.; Davis, M. E. J. Am. Chem. Soc. 1995, 117, 3766-3779.
(18) Mathew, T.; Elangovan, S. P.; Yokoi, T.; Tatsumi, T.; Ogura, M.; Kubota, Y.; Shimojima, A.; Okubo, T. Micropor. Mesopor. Mater. 2010, 129, 126-135.
(19) Elangovan, S. P.; Ogura, M.; Davis, M. E.; Okubo, T. J. Phys. Chem. B 2004, 108, 13059-13061.
(20) Bjørgen, M.; Svelle, S.; Joensen, F.; Nerlov, J.; Kolboe, S.; Bonino, F.; Palumbo, L.; Bordiga, S.; Olsbye, U. J. Catal. 2007, 249, 195-207.



