Latest Research
- 2017.07.05
- Yamamoto-Imaoka Group
Polymerization of Atom-Mimicking Dendrimer
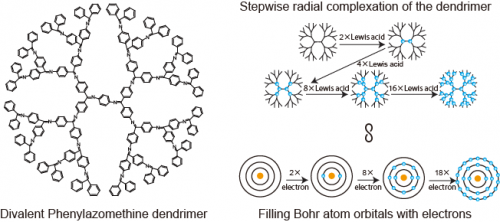
Atom-like handling of nano-objects, i.e., connecting macromolecules or nano-particles1 with a definite "valency" and "direction" is currently an objective of science. Recently, the layer-by-layer periodically branched structure of the dendrimer is getting attention for atom-mimicry2, because each dendrimer generation level looks like the traditional Bohr atomic orbital. Phenylazomethine (DPA) is a Schiff base dendritic ligand that coordinates to Lewis acids in a radial stepwise fashion3. This behavior is an analogue of filling the atomic orbital with electrons, and DPA definitely shows "atom mimicry" (Fig.1). We now report the first examples of the 1D and 2D polymers of DPA by selectively crosslinking the innermost layer (first orbital) of DPA which is the first "polymer of atom mimicry".
To crosslink DPA, a rod with two Lewis acids at the periphery is necessary. The rod is required to be rigid to prevent the two Lewis acids from one rod coordinating to one dendrimer, and the Lewis acid has to be synthetically connectable to the rod. Therefore, the combination of the phenylene ethynylene backbone and triphenylmethylium cation (TPM: Lewis acid)4 was selected as a linker which is an analogue of the covalent electron pair.
Figure1 (Left)Structure of disubstituted phenylazomethine dendrimer.(Right)Stepwise radial complexation as an analog of Bohr atom model.
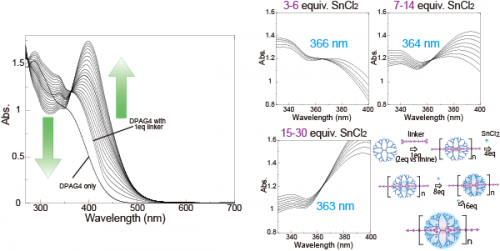
The coordination of the linker molecule to the innermost layer of the dendrimer was confirmed by Uv-vis titration. The additional titration of SnCl2 (a Lewis acid that has a lower binding constant to DPA compared to TPM) to the 1: 1 mixture of DPAG4 and the linker (Fig.2). First, the addition of 1eq of the linker (2eq cation vs. one dendrimer) caused an increase in the absorbance at 350-450 nm. Subsequently, SnCl2 was titrated into this solution. Upon the addition of SnCl2, the absorption around 450 nm increased and the absorption around 300 nm decreased. Careful observation revealed the existence of three isosbestic points, and the amount of SnCl2 that was necessary for the shift was 4, 8, and 16 equivalents. This corresponds to the number of imine sites of the second, third, and fourth layers of DPAG4, respectively and agrees with the stepwise complexation from the second layer to the fourth layer of DPAG4. Consequently, the UV-vis titration shows that the linker is coordinating to the innermost layer of the DPA, forming a coordination polymer, and the DPA polymer can store other Lewis acids.
Figure 2 UV-vis spectra during titration of phenylazomethine dendrimer (DPAG4) during the addition of 1 eq of the linker followed by 28 eq of SnCl2.
The structure of the dendrimer polymer was analyzed by microscopy (Fig.3). The solution of the 1:1 mixture of DPAG4 and the linker was cast onto a substrate. Fibrous objects that can be attributed to a bundling of the polymers were observed. Further determination by STM revealed the existence of a single chain with a width of 3nm that corresponds to the short axis of the dendrimer. The observed image also had a periodic structure that can be attributed to the dendrimer unit. These supramolecular polymer formation could also be observed with a tetra substituted phenylazomethine dendrimers that provided a sheet like structure.5
A supramolecular polymer that consists of an atom-mimicry (DPA) and an electron pair-mimicry (linker molecule) was developed. The DPA exhibiting atom-mimicry could store other Lewis acids in its outer orbitals. This means that DPA can align other materials that are incorporated6. This describes a new aspect of atom-mimicry, i.e., the use of atom-mimicking nano-containers to give atomicity to various molecules that can be stored in the container.
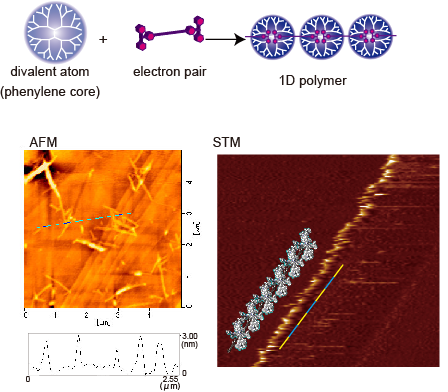
Figure 3 AFM and STM image of dendrimer-linker complex (polymer).
1 D. E. Bergeron, P. J. Roach, A. W. Castleman Jr., N. O. Jones, S. N. Khanna, Science, 307, 231 (2005).
2 D. A. Tomalia, S. N. Khanna, Chem. Rev., 116, 2705 (2016).
3 K. Yamamoto, M. Higuchi, S. Shiki, M. Tsuruta, H. Chiba, Nature, 415, 509 (2002).
4 Y. Ochi, A. Fujii, R. Nakajima, K. Yamamoto, Macromolecules, 43, 6570 (2010).
5 K. Albrecht, Y. Hirabayashi, M. Otake, S. Mendori, Y. Tobari, Y. Azuma, Y. Majima, K. Yamamoto, Sci. Adv., 2, e1601414 (2016).
6 K. Yamamoto, T. Imaoka, Acc. Chem. Res., 47, 1127 (2014)