Latest Research
- 2015.10.15
Synthesis and functionalities of Transition metal complexes bearing a linear-shape multidentate ligand
Transition metal complexes which are composed of metal ions and organic and/or inorganic ligands have been widely studied, because they often show characteristic physical and chemical properties and functions which cannot achieve only by metals or organic/inorganic compounds. A ligand which has multiple coordination sites can form various metal-ligand bond formations, therefore, metal complexes which have various structures can be formed, and such a complex are expected to show characteristic nature and behaviors different from metal complexes formed by monodentate lkigands.
1,9,10-Anthyridine (Scheme 1) can be named as such a compound. It has a rigid anthracene-type structure, and containing three imine nitrogens to be coordinatable to metal ions.
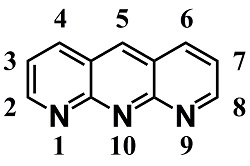
Scheme 1. 1,9,10-anthyridine
From such structural properties, a metal complex bearing 1,9,10-anthyridines are expected to form various structures as shown in Scheme 2. However, there have been only a few reports about the metal complexes having 1,9,10-anthyridines. [1]
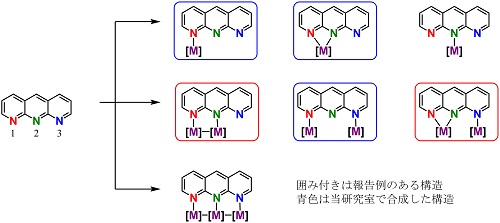
Scheme 2.
We synthesize new transition metal complexes having 1,9,10-anthyridine derivatives as a ligand, and investigate the physical/chemical properties and behaviors of the complexes. Our recent researches for development of molecular devices using multi-coordinativity, redox properties, emission properties of 1,9,10-anthyridines are introduced.
Dynamic behavior of 1,9,10-anthyridine-ligated Ru complex [Ru(bpy)2(dbanth)](PF6)2[2]
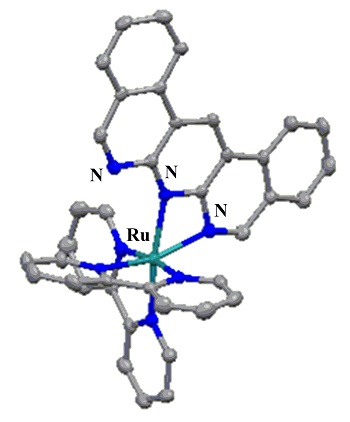
Dibenzo[c,h]anthyridine (dbanth), which is a derivative of 1,9,10-anthyridine, ligated Ru(II) complex, [Ru(bpy)2(dbanth)](PF6)2 ([1](PF6)2) was synthesized and the molecular structure was determined. In [1](PF6)2, the dbanth ligand is coordinated to Ru center by two imine nitrogens as shown in Figure 1.
Figure 1. Molecular structure of [1]2
The N-Ru-N bond angle of [1](PF6)2 is ca. 63º, which is more acute than 90º, indicating that the chelate coordination is distorted. The acetone solution of [1](PF6)2 shows brown color. On the other hand, when [1](PF6)2 is dissolved in acetonitrile, the color of the solution shows yellow (Figure 2).
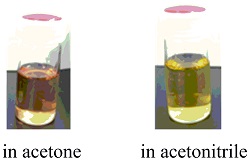
Figure 2.
We made crystals of [1](PF6)2 from acetonitrile solution, and determined the structure by X-ray Crystallography. As a result, the molecular structure of the obtained complex is monodentate coordinated dbanth-Ru complex, [Ru(bpy)2(dbanth)(CH3CN)](PF6)2 ([2](PF6)2) as shown in Figure 3.
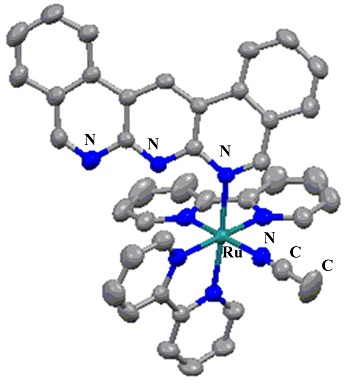
Figure 3. Molecular structure
In [2](PF6)2, the dbanth ligand is coordinated to the Ru center by one nitrogen atom, and one CH3CN molecule is also coordinated.
When [2](PF6)2 is dissolved in acetone, [1](PF6)2 is recovered smoothly. These results indicate that interconversion between [1](PF6)2 and [2](PF6)2 can be possible by treatment of solvents (Scheme 3).
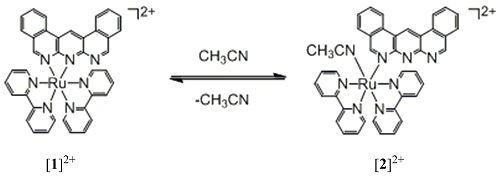
Scheme 3.
In addition, we found the characteristic dynamic behavior of the complex. When the behavior of the complex in the solution was investigated in detail, the change of the coordination site can be observed. The mechanism of the site-exchange reaction is shown in Scheme 4. The site change is achieved by coordination of acetonitrile - dissociation of acetonitrile - re-coordination of acetonitrile, and the monodentate-coordinated dbanth intermediate, it is considered that the dbanth may be coordinated to Ru center by central imine nitrogen, play an important role for the site exchange.
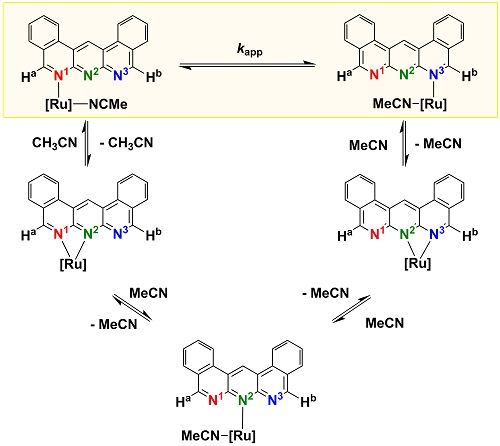
Scheme 4.
Plausible Dynamic processes for dbanth coordinated to Ru.
As mentioned above, we clarified the characteristic dynamic behavior of dbanth-ligated Rucomplex [1](PF6)2. [1](PF6)2 also shows interesting spectral properties. These properties come from the structure of dbanth, which is the formation ability of distorted chelate fashion, and reversible metal-ligand coordination bond. In the future, we would like to develop the complex as a dynamic molecular devices and synthesize new functional materials using the complex as an element block.
Acknowledgement: This study is partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas "New Polymeric Materials Based on Element-Blocks (No.2401)" (24102003) of The Ministry of Education, Culture, Sports, Science, and Technology, Japan.
[1] (a) S. -M. Peng et al., New J. Chem. 2012, 36, 2340.
(b) S. -T. Liu et al., Organometallics. 2013, 32, 4009.
(c) S. -T. Liu et al., J. Chinese Chem. Soc. 2013, 60, 839.
(d) M. Yagi et al., Inorg. Chem. 2015, 54, 7627.
[2] S. Hirakawa, T. Koizumi, Inorg. Chem. 2014, 53, 10788.