Latest Research
- 2022.08.24
- Yamaguchi-Kuroki Group
Comprehensive Design Strategy of Iron-based Electrocatalysts for Hydrogen Production Based on Experiment, First-principle Calculation, and Data Science
Current global energy issues have increasingly stimulated the demand for the innovation of alternative energy technologies that can satisfy net zero emission of carbon. Renewable energy resources, such as solar and wind energy sources, are very promising candidates to actualize the decarbonized society. One of examples of harnessing renewable energy-derived electricity is the splitting of earth-abundant water into hydrogen, which is a promising carbon-free chemical fuel with a high weight energy density. In contrast to polymer electrolyte water electrolysis in acidic environment, electrochemical alkaline water splitting can employ inexpensive nonprecious metals as electrocatalysts and bipolar plates, etc., which is favorable to apply this technology to a large-scale industrial process. We recently developed highly durable all-aromatic anion conducting polyelectrolytes for anion-exchange membranes in alkaline water electrolysis that were stable in electrolysis operation at 80 °C for longer than 150 h,[1] as highlighted in previous "Latest Research" in our institution (http://www.res.titech.ac.jp/english/news/reserch/202101.html). However, the anodic oxygen evolution reaction (OER) in the water splitting currently has a large overpotential, and it is a great bottleneck for widespread use. Thus, highly active and inexpensive electrocatalysts for OER are desired to overcome this concern.
Iron (Fe) is a very earth-abundant element that is a quite cheap[2] and non-toxic metal, and Fe-based electrocatalysts have thus recently received extensive interest. Because simple Fe compounds intrinsically have poor OER performance, some previous studies fabricated multimetal electrocatalysts containing Fe and other metals to improve the OER performance. However, most of the previous studies focused on the choice of elements, and these OER activities were modulated by the elemental compositions in the catalysts. In contrast, the influence of crystal structures on OER activity have not yet been argued enough.
To investigate the influence of crystal structures on electrocatalytic OER efficiencies on Fe-based oxides overall, we collected OER activities of Fe-based simple and bimetal oxides from our experiments[3] and reported papers,[4] and their structural characteristics were obtained from a structure database for inorganic compounds (ICSD). Fig. 1a overviews the investigated Fe-based oxides in this study. Thus, we found a clear correlation of OER efficiencies on the Fe-based oxides with minimum Fe-O bond lengths in their bulk crystals; namely, a shorter Fe-O bond length resulted in a higher OER activity, as shown in Fig. 1b. Meanwhile, OER efficiencies did not exhibit clear correlations with other structural characteristics, minimum Fe-O-Fe bond angle, minimum Fe-Fe interatomic distance, fraction of edge- or face-shared connectivity of FeOx polyhedra and the maximum distortion index, as shown in Fig. 1c-f.[3a]
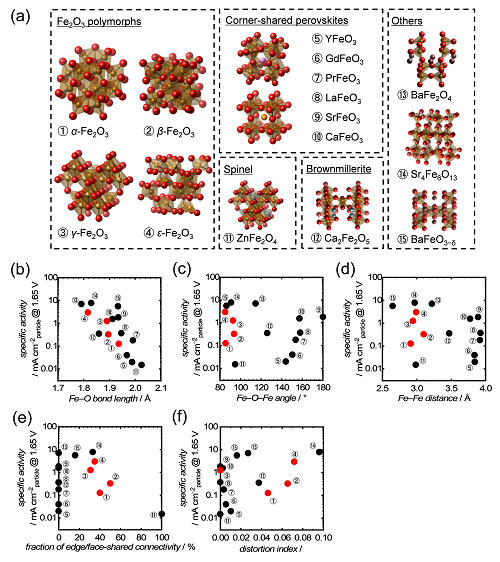 |
|
| Fig. 1 |
(a) Crystal structures of the collected Fe-based oxides. OER activities of the Fe2O3 polymorphs[3a] and reported Fe-based oxide OER electrocatalysts from the literature[3b, 4] at 1.65 V as a function of (b) the minimum Fe-O bond length, (c) the minimum Fe-O-Fe bond angle, (d) the minimum Fe-Fe interatomic distance, (e) fraction of edge- or face-shared connectivity of FeOx polyhedra in the crystals, and (f) the maximum distortion index of FeOx polyhedra.[3a] Copyright 2021 Wiley‐VCH GmbH. Reproduced with permission from Y. Sugawara, K. Kamata, E. Hayashi, M. Itoh, Y. Hamasaki, T. Yamaguchi, Comprehensive Structural Descriptor for Electrocatalytic Oxygen Evolution Activities of Iron Oxides, ChemElectroChem, John Wiley and Sons.
|
According to the identified relationship between OER activity and crystal structure, we subsequently attempted to rapidly find a novel outstanding Fe-based OER electrocatalysts. The aforementioned structure-activity relationship strongly suggested that Fe-based oxides with the shortest Fe-O bond length should catalyze OER with the highest efficiency. Therefore, we used structure databases to mine potential candidates and chose unreported Fe-based bimetal and trimetal oxides with various structures, which can significantly accelerate the catalyst developments. Fig. 2 overviews the chosen Fe-based oxides that consists of two or three metallic elements, and these oxides possess very complicated crystal structures: M-type hexaferrites, Ruddlesden-Poppers, brownmillerite and stuffed tridymite. These nine kinds of Fe-based oxides have never been evaluated as OER catalysts in alkaline. Among these Fe-based oxides, Ba0.65Ca0.35Fe12O19 contains very short Fe-O bond length, 1.552 Å, which was expected to exhibit a very prominent OER efficiency. We then fabricated the Fe-based multimetal oxides and measured their OER activity in alkaline conditions. Notably, the OER efficiencies on the unreported Fe-based multimetal oxides also exhibited great correlation with Fe-O bond lengths, as presented in Fig. 3. It is noteworthy that the trend in the OER efficiencies and the Fe-O bond lengths is applicable to a wide range of Fe-based simple, bimetal, and trimetal oxides regardless of elemental compositions, crystal categories, and Fe valence states.[5] In addition, Ba0.65Ca0.35Fe12O19 displayed very prominent OER activity due to its very short Fe-O bond length, as we expected.
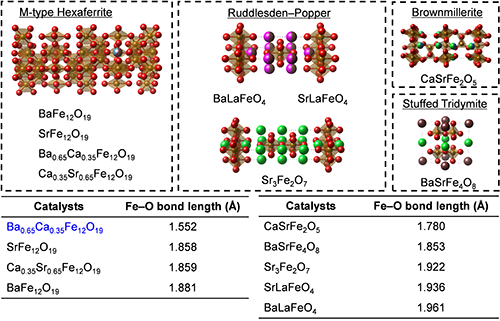 |
|
| Fig. 2 |
Crystal structures of unreported iron-based multimetal oxides that were evaluated in this study.[5] Copyright 2022 Wiley‐VCH GmbH.Adapted with permission from Y. Sugawara, S. Ueno, K. Kamata, T. Yamaguchi, Crystal Structures of Iron-Based Oxides and Their Catalytic Efficiencies for the Oxygen Evolution Reaction: A Trend in Alkaline Media, ChemElectroChem, John Wiley and Sons.
|
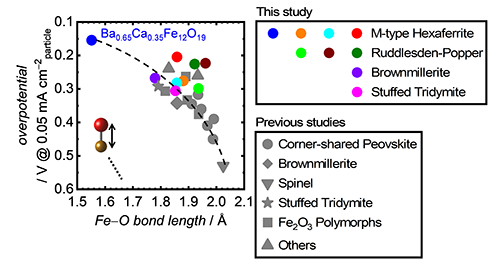 |
|
| Fig. 3 |
OER overpotentials on Fe-based oxides as a function of Fe−O bond length. The colorful markers were obtained data from the unreported Fe-based multimetal oxides, which were examined for the first time in the current study.[5] The data highlighted by the gray color were collected from previous studies.[3,4] Copyright 2022 Wiley‐VCH GmbH. Adapted with permission from Y. Sugawara, S. Ueno, K. Kamata, T. Yamaguchi, Crystal Structures of Iron-Based Oxides and Their Catalytic Efficiencies for the Oxygen Evolution Reaction: A Trend in Alkaline Media, ChemElectroChem, John Wiley and Sons.
|
Furthermore, machine learning (ML) analysis also applied to the aforementioned Fe-based oxide electrocatalysts to identify the most dominant structural descriptor for OER activity. We have ranked the relative importance of the structural characteristics for OER: Fe-O bond length for OER effciencies, Fe-Fe interatomic distance, Fe-O-Fe bond angle and distortion index using six types of (non)linear regressions. Consequently, Fe-O bond length possessed the highest relative importance for OER overpotentials according to a fitted model using random forest regression (RFR), as shown in Fig. 4.[5] Therefore, the ML results concluded that Fe−O bond length is the most dominant structural factor for the electrocatalytic OER efficiency on Fe-based oxides. These findings demonstrated that Fe−O bond length is a comprehensive, reliable and easily available structural descriptor for OER efficiency on Fe-based oxides and can accelerate rapid innovation of OER electrocatalysts for hydrogen production.
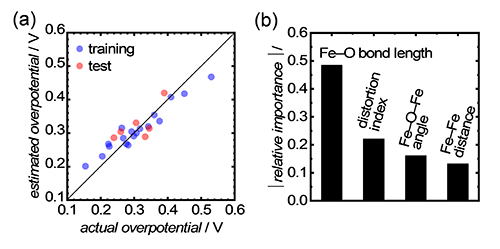 |
|
| Fig. 4 |
(a) Plots of actual and estimated OER overpotentials using a fitted model by random forrest regression (RFR). (b) Output relative importance for OER overpotential from RFR for the four kinds of structural descriptors.[5] Copyright 2022 Wiley‐VCH GmbH. Adapted with permission from Y. Sugawara, S. Ueno, K. Kamata, T. Yamaguchi, Crystal Structures of Iron-Based Oxides and Their Catalytic Efficiencies for the Oxygen Evolution Reaction: A Trend in Alkaline Media, ChemElectroChem, John Wiley and Sons.
|
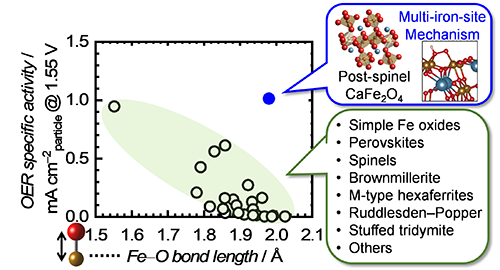
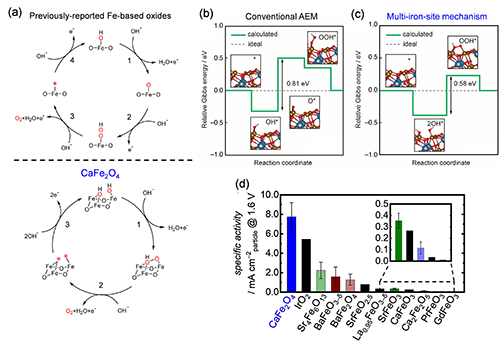
However, it is desirable to surpass the foregoing structure-activity relationship because Fe-O bond length in Fe-based oxides is basically limited, and more active OER electrocatalysts cannot be achieved according to this guide. Therefore, we try to switch the reaction mechanism for OER on a Fe-based oxide by the structural modulation around Fe atoms because different mechanisms can be driven by different factors; i.e., the mechanism switch could overcome the foregoing structure-activity relationship. Thus, we unveiled post-spinel CaFe2O4 that bears a much amount of edge-shared FeO6 connectivity in its structure. It is remarkable that CaFe2O4 exhibited prominent OER activity despite long Fe-O bond length in its crystal, as plotted in Fig. 5, and the OER electrocatalysis on CaFe2O4 clearly overcame the foregoing structure-activity relationship. To elucidate the outstanding OER efficiency on CaFe2O4, we carried out first-principle calculations and uncovered that the structural characteristics of CaFe2O4 enables to promote an unprecedented OER mechanism, multi-iron-site mechanism, where OER process can be facilitated through direct O-O bond formation on three Fe atoms as illustrated in Fig. 6a. The multi-iron-site mechanism can skip the rate-determining step of previously-reported mechanism, formation of O*, and reduce the energy barrier on the OER process, as shown in Fig. 6b and c. Consequently, the OER activity of CaFe2O4 was much higher than those of previously reported Fe-based multimetal oxides and even outperformed that of benchmark rare metal catalyst, IrO2, as exhibited in Fig. 6d.[3b]
 |
|
| Fig. 5 |
Relationship between OER specific activity of Fe-based oxides and Fe-O bond lengths in their crystals, including CaFe2O4 and other various categories of simple and multimetal oxides.[3,5]
|
 |
|
| Fig. 6 |
(a) Proposed Reaction Mechanism for the OER on CaFe2O4. Gibbs energy diagrams of the OER on CaFe2O4 with the (b) conventional adsorbate evolution mechanism (AEM) and (c) multi-iron-site mechanism calculated with the DFT. The calculated values and ideal values are shown in green and gray lines. The theoretical equilibrium potentials of the OER are applied for each mechanism. The insets show the optimized geometries of the reactant and intermediate states in the energy diagram. Theoretical overpotentials at the potential determining step are shown. (d) Comparison of the OER specific activity at 1.6 V of CaFe2O4 with those of IrO2 and the previously reported Fe-based oxides containing alkaline-earth and rare-earth metals. The inset chart is a magnification of the small bars in the graph.[3b] Reproduced with permission from Y. Sugawara, K. Kamata, A. Ishikawa, Y. Tateyama, T. Yamaguchi ACS Appl. Energy Mater. 2021, 4, 4, 3057-3066 Copyright © 2018 American Chemical Society.
|
In conclusion, our studies uncovered the comprehensive and reliable structural descriptor that determine OER efficiency on Fe-based multimetal oxides using experiments, first-principle calculations, and data-driven ML. In addition, the very active Fe-based catalyst, CaFe2O4, was successfully developed by modulation of crystal structures; consequently, the best OER activity among Fe-based oxides were achieved and even outperformed IrO2. These findings can contribute to the innovation of electrochemical hydrogen production.
The part of this paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
| [1] | R. Soni, S. Miyanishi, H. Kuroki, T. Yamaguchi, ACS Appl. Energy Mater. 2021,4, 1053−1058. |
| [2] | P. C. K. Vesborg, T. F. Jaramillo, RSC Adv. 2012, 2, 7933-7947. |
| [3] | a) Y. Sugawara, K. Kamata, E. Hayashi, M. Itoh, Y. Hamasaki, T. Yamaguchi,ChemElectroChem 2021, 8, 4466-4471; b) Y. Sugawara, K. Kamata, A. Ishikawa, Y. Tateyama, T. Yamaguchi, ACS Appl. Energy Mater. 2021, 4, 3057-3066. |
| [4] | a) H. Y. Li, Y. B. Chen, S. B. Xi, J. X. Wang, S. N. Sun, Y. M. Sun, Y. H. Du, Z. C. J. Xu, Chem. Mater. 2018, 30, 4313-4320; b) I. Yamada, A. Takamatsu, K. Asai, T. Shirakawa, H. Ohzuku, A. Seno, T. Uchimura, H. Fujii, S. Kawaguchi, K. Wada, H. Ikeno, S. Yagi, J. Phys. Chem. C 2018, 122, 27885-27892. |
| [5] | Y. Sugawara, S. Ueno, K. Kamata, T. Yamaguchi, ChemElectroChem 2022, 9,e202101679. |



